Personalized tissue-engineered arteries as vascular graft transplants: A safety study in sheep
- PMID: 36110971
- PMCID: PMC9463533
- DOI: 10.1016/j.reth.2022.08.005
Personalized tissue-engineered arteries as vascular graft transplants: A safety study in sheep
Abstract
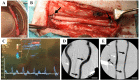
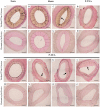
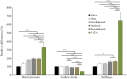
Patients with cardiovascular disease often need replacement or bypass of a diseased blood vessel. With disadvantages of both autologous blood vessels and synthetic grafts, tissue engineering is emerging as a promising alternative of advanced therapy medicinal products for individualized blood vessels. By reconditioning of a decellularized blood vessel with the recipient's own peripheral blood, we have been able to prevent rejection without using immunosuppressants and prime grafts for efficient recellularization in vivo. Recently, decellularized veins reconditioned with autologous peripheral blood were shown to be safe and functional in a porcine in vivo study as a potential alternative for vein grafting. In this study, personalized tissue engineered arteries (P-TEA) were developed using the same methodology and evaluated for safety in a sheep in vivo model of carotid artery transplantation. Five personalized arteries were transplanted to carotid arteries and analyzed for safety and patency as well as with histology after four months in vivo. All grafts were fully patent without any occlusion or stenosis. The tissue was well cellularized with a continuous endothelial cell layer covering the luminal surface, revascularized adventitia with capillaries and no sign of rejection or infection. In summary, the results indicate that P-TEA is safe to use and has potential as clinical grafts.
Keywords: ATMP; Blood vessels; Recellularization; Regenerative medicine; Scaffold; Tissue engineering.
© 2022 The Japanese Society for Regenerative Medicine. Production and hosting by Elsevier B.V.
Conflict of interest statement
Lachmi Jenndahl, Tobias Gustafsson-Hedberg, Robin Simsa and Raimund Strehl were employees of the company VERIGRAFT AB which contributed financially, including salaries and study costs, and by providing laboratory space.
Figures


References
-
- Cines D.B., Pollak E.S., Buck C.A., Loscalzo J., Zimmerman G.A., McEver R.P., et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91(10):3527–3561. - PubMed
-
- Conte M.S., Bandyk D.F., Clowes A.W., Moneta G.L., Seely L., Lorenz T.J., et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43(4):742–751. doi: 10.1016/j.jvs.2005.12.058. discussion 751. - DOI - PubMed
LinkOut - more resources
Full Text Sources

