A silk fibroin/chitosan/nanohydroxyapatite biomimetic bone scaffold combined with autologous concentrated growth factor promotes the proliferation and osteogenic differentiation of BMSCs and repair of critical bone defects
- PMID: 36110973
- PMCID: PMC9459434
- DOI: 10.1016/j.reth.2022.08.006
A silk fibroin/chitosan/nanohydroxyapatite biomimetic bone scaffold combined with autologous concentrated growth factor promotes the proliferation and osteogenic differentiation of BMSCs and repair of critical bone defects
Abstract
Purpose: With the goal of increasing the translational efficiency of bone tissue engineering for practical clinical applications, biomimetic composite scaffolds combined with autologous endogenous growth factors for repairing bone defects have become a current research hotspot. In this study, we prepared a silk fibroin/chitosan/nanohydroxyapatite (SF/CS/nHA) composite biomimetic scaffold and then combined it with autologous concentrated growth factor (CGF) to explore the effect of this combination on the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) and the efficiency of repairing critical radial defects.
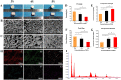
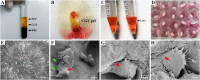
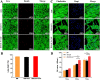
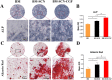
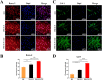
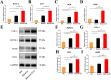
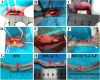
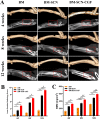
Methods: Three kinds of SF/CS/nHA composite biomimetic scaffolds with mass fractions of 3%, 4%, and 5% were prepared by vacuum freeze-drying and chemical cross-linking methods, and the characteristics of the scaffolds were evaluated. In vitro, BMSCs were seeded on SF/CS/nHA scaffolds, and then CGF was added. The morphology and proliferation of BMSCs were evaluated by live-dead staining, phalloidin staining, and CCK-8 assays. ALP staining, alizarin red staining, cellular immunofluorescence, RT-PCR, and Western blotting were used to detect the osteogenic differentiation of BMSCs. In vivo, a rabbit radius critical bone defect model was constructed, and the SF/CS/nHA-BMSC scaffold cell complex combined with CGF was implanted. The effect on bone defect repair was evaluated by 3D CT scanning, HE staining, Masson staining, and immunohistochemistry.
Results: The characteristics of 4% SF/CS/nHA were the most suitable for repairing bone defects. In vitro, the SF/CS/nHA combined CGF group showed better adhesion, cell morphology, proliferation, and osteogenic differentiation of BMSCs than the other groups (P < 0.05 for all). In vivo imaging examination and histological analysis demonstrated that the SF/CS/nHA scaffold combined with CGF had better efficiency in bone defect repair than the other scaffolds (P < 0.05 for all).
Conclusions: A SF/CS/nHA composite biomimetic bone scaffold combined with autologous CGF promoted the proliferation and osteogenic differentiation of BMSCs in vitro and improved the repair efficiency of critical bone defects in vivo. This combination may have the potential for clinical translation due to its excellent biocompatibility.
Keywords: Biomimetic bone scaffold; Bone marrow mesenchymal stem cells (BMSCs); Bone regeneration; Concentrated growth factor (CGF).
© 2022 The Japanese Society for Regenerative Medicine. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Icariin-loaded composite scaffold promotes osteogenic differentiation and bone regeneration.BMC Musculoskelet Disord. 2025 Jun 3;26(1):548. doi: 10.1186/s12891-025-08824-4. BMC Musculoskelet Disord. 2025. PMID: 40462026 Free PMC article.
-
Polydopamine-coated biomimetic bone scaffolds loaded with exosomes promote osteogenic differentiation of BMSC and bone regeneration.Regen Ther. 2023 Mar 30;23:25-36. doi: 10.1016/j.reth.2023.03.005. eCollection 2023 Jun. Regen Ther. 2023. PMID: 37063095 Free PMC article.
-
Composite scaffolds loaded with bone mesenchymal stem cells promote the repair of radial bone defects in rabbit model.Biomed Pharmacother. 2018 Jan;97:600-606. doi: 10.1016/j.biopha.2017.10.110. Epub 2017 Nov 6. Biomed Pharmacother. 2018. PMID: 29101803
-
Silk fibroin scaffolds: A promising candidate for bone regeneration.Front Bioeng Biotechnol. 2022 Nov 25;10:1054379. doi: 10.3389/fbioe.2022.1054379. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36507269 Free PMC article. Review.
-
Recent developments in biomaterials for long-bone segmental defect reconstruction: A narrative overview.J Orthop Translat. 2019 Oct 8;22:26-33. doi: 10.1016/j.jot.2019.09.005. eCollection 2020 May. J Orthop Translat. 2019. PMID: 32440496 Free PMC article. Review.
Cited by
-
Biomaterial-Based Additive Manufactured Composite/Scaffolds for Tissue Engineering and Regenerative Medicine: A Comprehensive Review.Polymers (Basel). 2025 Apr 17;17(8):1090. doi: 10.3390/polym17081090. Polymers (Basel). 2025. PMID: 40284355 Free PMC article. Review.
-
β-tricalcium phosphate/gelatin composite scaffolds incorporated with gentamycin-loaded chitosan microspheres for infected bone defect treatment.PLoS One. 2022 Dec 8;17(12):e0277522. doi: 10.1371/journal.pone.0277522. eCollection 2022. PLoS One. 2022. PMID: 36480529 Free PMC article.
-
Chitosan alchemy: transforming tissue engineering and wound healing.RSC Adv. 2024 Jun 17;14(27):19219-19256. doi: 10.1039/d4ra01594k. eCollection 2024 Jun 12. RSC Adv. 2024. PMID: 38887635 Free PMC article. Review.
-
Bone Morphogenetic Protein 7-Loaded Gelatin Methacrylate/Oxidized Sodium Alginate/Nano-Hydroxyapatite Composite Hydrogel for Bone Tissue Engineering.Int J Nanomedicine. 2024 Jun 25;19:6359-6376. doi: 10.2147/IJN.S461996. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38946885 Free PMC article.
-
Polyurethane/silk fibroin-based electrospun membranes for wound healing and skin substitute applications.Beilstein J Nanotechnol. 2025 Apr 24;16:591-612. doi: 10.3762/bjnano.16.46. eCollection 2025. Beilstein J Nanotechnol. 2025. PMID: 40297246 Free PMC article. Review.
References
-
- Fu Z., Cui J., Zhao B., Shen S.G., Lin K. An overview of polyester/hydroxyapatite composites for bone tissue repairing. J Orthop Translat. 2021;28:118–130. https://doi:10.1016/j.jot.2021.02.005 - DOI - PMC - PubMed
-
- Song T., Zhou J., Shi M., Xuan L., Jiang H., Lin Z., et al. Osteon-mimetic 3D nanofibrous scaffold enhances stem cell proliferation and osteogenic differentiation for bone regeneration. Biomater Sci. 2022;10:1090–1103. https://doi:10.1039/d1bm01489g - DOI - PubMed
-
- Wang L., Wei X., Duan C., Yang J., Xiao S., Liu H., et al. Bone marrow mesenchymal stem cell sheets with high expression of hBD3 and CTGF promote periodontal regeneration. Mater Sci Eng C Mater Biol Appl. 2022 https://doi:10.1016/j.msec.2022.112657 - DOI - PubMed
-
- Grande F., Tucci P. Titanium dioxide nanoparticles: a risk for human Health? Mini Rev Med Chem. 2016;16:762–769. https://doi:10.2174/1389557516666160321114341 - DOI - PubMed
-
- Han X., Zhou X., Qiu K., Feng W., Mo H., Wang M., et al. Strontium-incorporated mineralized PLLA nanofibrous membranes for promoting bone defect repair. Colloids Surf B Biointerfaces. 2019;179:363–373. https://doi:10.1016/j.colsurfb.2019.04.011 - DOI - PubMed
LinkOut - more resources
Full Text Sources

