Appropriate pore size for bone formation potential of porous collagen type I-based recombinant peptide
- PMID: 36110974
- PMCID: PMC9445290
- DOI: 10.1016/j.reth.2022.08.001
Appropriate pore size for bone formation potential of porous collagen type I-based recombinant peptide
Abstract
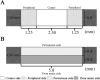
Introduction: In this study, we developed porous medium cross-linked recombinant collagen peptide (mRCP) with two different ranges of interconnected pore sizes, Small-mRCP (S-mRCP) with a range of 100-300 μm and Large-mRCP (L-mRCP) with a range of 200-500 μm, to compare the effect of pore size on bone regeneration in a calvarial bone defect.
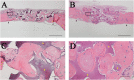
Methods: Calvarial bone defects were created in Sprague-Dawley rats through a surgical procedure. The rats were divided into 2 groups: S-mRCP implanted group and L-mRCP implanted group. The newly formed bone volume and bone mineral density (BMD) was evaluated by micro-computed tomography (micro-CT) immediately after implantation and at 1, 2, 3, and 4 weeks after implantation. In addition, histological analyses were carried out with hematoxylin and eosin (H&E) staining at 4 weeks after implantation to measure the newly formed bone area between each group in the entire defect, as well as the central side, the two peripheral sides (right and left), the periosteal (top) side and the dura matter (bottom) side of the defect.
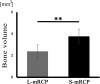
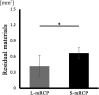
Results: Micro-CT analysis showed no significant differences in the amount of bone volume between the S-mRCP and L-mRCP implanted groups at 1, 2, 3 and 4 weeks after implantation. BMD was equivalent to that of the adjacent native calvaria bone at 4 weeks after implantation. H&E images showed that the newly formed bone area in the entire defect was significantly larger in the S-mRCP implanted group than in the L-mRCP implanted group. Furthermore, the amount of newly formed bone area in all sides of the defect was significantly more in the S-mRCP implanted group than in the L-mRCP implanted group.
Conclusion: These results indicate that the smaller pore size range of 100-300 μm is appropriate for mRCP in bone regeneration.
Keywords: ALP, alkaline phosphatase; BMD, bone mineral density; Bone reconstruction; Bone substitute; CSD, critical-size defect; Calvaria; DHT, dehydothermal treatment; H&E, hematoxylin and eosin; Interconnected pore; Pore size; RCP, recombinant collagen peptide; RGD, arginyl- glycyl- aspartic acid; ROIs, regions of interest; Recombinant human collagen peptide; SD, standard deviation; TRAP, tartrate-resistant acid phosphatase; mRCP, medium-cross-linked RCP; micro-CT, micro-computed tomography.
© 2022 The Japanese Society for Regenerative Medicine. Production and hosting by Elsevier B.V.
Conflict of interest statement
This work was supported by the joint research expenses with 10.13039/501100013899FUJIFILM Corporation. Taku Wakita, Takahiro Hiratsuka are employees of FUJIFILM Corporation.
Figures








Similar articles
-
Collagen type I-based recombinant peptide promotes bone regeneration in rat critical-size calvarial defects by enhancing osteoclast activity at late stages of healing.Regen Ther. 2023 Oct 6;24:515-527. doi: 10.1016/j.reth.2023.09.013. eCollection 2023 Dec. Regen Ther. 2023. PMID: 37841660 Free PMC article.
-
Bone formation potential of collagen type I-based recombinant peptide particles in rat calvaria defects.Regen Ther. 2020 Dec 24;16:12-22. doi: 10.1016/j.reth.2020.12.001. eCollection 2021 Mar. Regen Ther. 2020. PMID: 33426238 Free PMC article.
-
Effect of Resorbable Collagen Plug on Bone Regeneration in Rat Critical-Size Defect Model.Implant Dent. 2016 Apr;25(2):163-70. doi: 10.1097/ID.0000000000000396. Implant Dent. 2016. PMID: 26901636
-
Evaluation of the osteogenic potential of demineralized and decellularized bovine bone granules following implantation in rat calvaria critical-size defect model.PLoS One. 2023 Dec 21;18(12):e0294291. doi: 10.1371/journal.pone.0294291. eCollection 2023. PLoS One. 2023. PMID: 38127838 Free PMC article.
-
Guided bone regeneration in standardized calvarial defects using beta-tricalcium phosphate and collagen membrane: a real-time in vivo micro-computed tomographic experiment in rats.Odontology. 2016 May;104(2):199-210. doi: 10.1007/s10266-015-0211-8. Epub 2015 Jul 9. Odontology. 2016. PMID: 26156449
Cited by
-
Composite Polylactide/Polycaprolactone Foams with Hierarchical Porous Structure for Pre-Vascularized Tissue Engineering.Int J Mol Sci. 2025 Mar 25;26(7):2974. doi: 10.3390/ijms26072974. Int J Mol Sci. 2025. PMID: 40243624 Free PMC article.
-
Collagen type I-based recombinant peptide promotes bone regeneration in rat critical-size calvarial defects by enhancing osteoclast activity at late stages of healing.Regen Ther. 2023 Oct 6;24:515-527. doi: 10.1016/j.reth.2023.09.013. eCollection 2023 Dec. Regen Ther. 2023. PMID: 37841660 Free PMC article.
References
-
- Bajaj A.K., Wongworawat A.A., Punjabi A. Management of alveolar clefts. J Craniofac Surg. 2003;14:840. - PubMed
LinkOut - more resources
Full Text Sources

