Trastuzumab plus pertuzumab in combination with chemotherapy in metastatic HER2-positive breast cancer: a retrospective single-armed cohort study in China
- PMID: 36111041
- PMCID: PMC9469119
- DOI: 10.21037/atm-22-3592
Trastuzumab plus pertuzumab in combination with chemotherapy in metastatic HER2-positive breast cancer: a retrospective single-armed cohort study in China
Abstract
Background: There are currently few studies on the efficacy and safety of the dual human epidermal growth factor receptor 2 (HER2)-targeted combination of trastuzumab and pertuzumab in second-line or subsequent therapy of metastatic breast cancer (MBC). This study retrospectively demonstrated the clinical efficacy and side effects of trastuzumab plus pertuzumab in combination with chemotherapy in HER2-positive MBC.
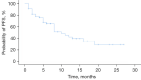
Methods: Patients with HER2-positive MBC and treated with trastuzumab plus pertuzumab combined with chemotherapy at our hospital between August 2013 and October 2021 were included. The primary endpoint was progression-free survival (PFS), and the secondary endpoints were disease control rate (DCR), objective response rate (ORR), clinical benefit rate (CBR), and side effects. PFS was calculated using the Kaplan-Meier method and side effects were assessed according to Common Terminology Criteria for Adverse Events 5.0.
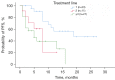
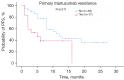
Results: A total of 55 women were included and the median PFS for trastuzumab plus pertuzumab combined with chemotherapy was 10 months. For the different treatment lines, the median PFS was 19, 8, and 5 months in first, second, and third and beyond, respectively. The DCR, ORR, and CBR were 81.8%, 47.3%, and 56.4%, respectively. The median PFS of patients with primary trastuzumab resistance was significantly shorter than trastuzumab-sensitive patients (5 vs. 12 months, P=0.011). The most common adverse reactions were neutropenia (40.0%), leukopenia (34.5%), thrombocytopenia (32.7%), and diarrhea (29.1%). The most common grade 3-4 adverse reactions were leukopenia (12.7%), thrombocytopenia (9.1%), and diarrhea (9.1%).
Conclusions: For patients with HER2-positive MBC, treatment with trastuzumab plus pertuzumab combined with chemotherapy appeared efficacious and safe.
Keywords: Trastuzumab; human epidermal growth factor receptor 2 positive (HER2-positive); metastatic breast cancer (MBC); pertuzumab; side effects.
2022 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3592/coif). The authors have no conflicts of interest to declare.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
