A nexus of lipid and O- Glcnac metabolism in physiology and disease
- PMID: 36111295
- PMCID: PMC9468787
- DOI: 10.3389/fendo.2022.943576
A nexus of lipid and O- Glcnac metabolism in physiology and disease
Abstract
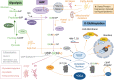
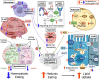
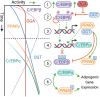
Although traditionally considered a glucose metabolism-associated modification, the O-linked β-N-Acetylglucosamine (O-GlcNAc) regulatory system interacts extensively with lipids and is required to maintain lipid homeostasis. The enzymes of O-GlcNAc cycling have molecular properties consistent with those expected of broad-spectrum environmental sensors. By direct protein-protein interactions and catalytic modification, O-GlcNAc cycling enzymes may provide both acute and long-term adaptation to stress and other environmental stimuli such as nutrient availability. Depending on the cell type, hyperlipidemia potentiates or depresses O-GlcNAc levels, sometimes biphasically, through a diversity of unique mechanisms that target UDP-GlcNAc synthesis and the availability, activity and substrate selectivity of the glycosylation enzymes, O-GlcNAc Transferase (OGT) and O-GlcNAcase (OGA). At the same time, OGT activity in multiple tissues has been implicated in the homeostatic regulation of systemic lipid uptake, storage and release. Hyperlipidemic patterns of O-GlcNAcylation in these cells are consistent with both transient physiological adaptation and feedback uninhibited obesogenic and metabolic dysregulation. In this review, we summarize the numerous interconnections between lipid and O-GlcNAc metabolism. These links provide insights into how the O-GlcNAc regulatory system may contribute to lipid-associated diseases including obesity and metabolic syndrome.
Keywords: O-GlcNAc; fatty acid; glycosylation; hexosamine biosynthetic pathway; homeostasis; lipid; metabolism; obesity.
Copyright © 2022 Lockridge and Hanover.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Zachara N, Akimoto Y, Hart GW. The O-GlcNAc modification. In: Essentials of glycobiology Cold spring harbor (NY): Cold Spring Harbor Laboratory Press; (2015). p. 239–51. rd.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

