Eupalinolide A induces autophagy via the ROS/ERK signaling pathway in hepatocellular carcinoma cells in vitro and in vivo
- PMID: 36111510
- PMCID: PMC9507091
- DOI: 10.3892/ijo.2022.5421
Eupalinolide A induces autophagy via the ROS/ERK signaling pathway in hepatocellular carcinoma cells in vitro and in vivo
Abstract
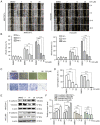
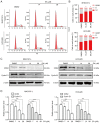
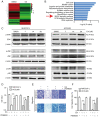
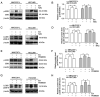
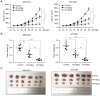
Hepatocellular carcinoma is the most common primary malignancy of the liver. The current systemic drugs used to treat hepatocellular carcinoma result in low overall survival time. It has therefore been suggested that new small‑molecule drugs should be developed for treating hepatocellular carcinoma. Eupatorium lindleyanum DC. (EL) has been used to treat numerous diseases, particularly respiratory diseases; however, to the best of our knowledge, studies have not yet fully elucidated the effect of EL on hepatocellular carcinoma. In the present study, the effect of eupalinolide A (EA), one of the extracts of EL, was evaluated on tumor growth in a xenograft model of human hepatocellular carcinoma cells, and on the proliferation and migration of hepatocellular carcinoma cell lines. Cell cycle progression and the type of cell death were then evaluated using the Cell Counting Kit 8 assay, flow cytometry, electron microscopy and western blotting. EA significantly inhibited cell proliferation and migration by arresting the cell cycle at the G1 phase and inducing autophagy in hepatocellular carcinoma cells. EA‑induced autophagy was mediated by reactive oxygen species (ROS) and ERK signaling activation. Specific inhibitors of ROS, autophagy and ERK inhibited EA‑induced cell death and migration. In conclusion, the present study revealed that EA may inhibit the proliferation and migration of hepatocellular carcinoma cells, highlighting its potential as a promising antitumor compound for treating hepatocellular carcinoma.
Keywords: ERK; autophagy; eupalinolide A; hepatocellular carcinoma; reactive oxygen species.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Eupalinolide B inhibits hepatic carcinoma by inducing ferroptosis and ROS-ER-JNK pathway.Acta Biochim Biophys Sin (Shanghai). 2022 Jul 25;54(7):974-986. doi: 10.3724/abbs.2022082. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 35866605 Free PMC article.
-
Tanshinone I induces cell apoptosis by reactive oxygen species-mediated endoplasmic reticulum stress and by suppressing p53/DRAM-mediated autophagy in human hepatocellular carcinoma.Artif Cells Nanomed Biotechnol. 2020 Dec;48(1):488-497. doi: 10.1080/21691401.2019.1709862. Artif Cells Nanomed Biotechnol. 2020. PMID: 32013613
-
A novel sesquiterpene lactone fraction from Eupatorium chinense L. suppresses hepatocellular carcinoma growth by triggering ferritinophagy and mitochondrial damage.Phytomedicine. 2023 Apr;112:154671. doi: 10.1016/j.phymed.2023.154671. Epub 2023 Jan 20. Phytomedicine. 2023. PMID: 36773432
-
Oxidative Stress-Driven Autophagy acROSs Onset and Therapeutic Outcome in Hepatocellular Carcinoma.Oxid Med Cell Longev. 2019 May 8;2019:6050123. doi: 10.1155/2019/6050123. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31205585 Free PMC article. Review.
-
New Drugs Effective in the Systemic Treatment of Hepatocellular Carcinoma.Clin Liver Dis (Hoboken). 2019 Sep 2;14(2):56-61. doi: 10.1002/cld.796. eCollection 2019 Aug. Clin Liver Dis (Hoboken). 2019. PMID: 31508221 Free PMC article. Review. No abstract available.
Cited by
-
Characterization of the metabolism of eupalinolide A and B by carboxylesterase and cytochrome P450 in human liver microsomes.Front Pharmacol. 2023 Jan 25;14:1093696. doi: 10.3389/fphar.2023.1093696. eCollection 2023. Front Pharmacol. 2023. PMID: 36762117 Free PMC article.
-
Transcriptomic analysis reveals pharmacological mechanisms mediating efficacy of Yangyinghuoxue Decoction in CCl4-induced hepatic fibrosis in rats.Front Pharmacol. 2024 May 15;15:1364023. doi: 10.3389/fphar.2024.1364023. eCollection 2024. Front Pharmacol. 2024. PMID: 38813108 Free PMC article.
-
Sesquiterpene Lactones as Promising Phytochemicals to Cease Metastatic Propagation of Cancer.Biomolecules. 2025 Feb 12;15(2):268. doi: 10.3390/biom15020268. Biomolecules. 2025. PMID: 40001571 Free PMC article. Review.
-
Sesquiterpene lactones as emerging biomolecules to cease cancer by targeting apoptosis.Front Pharmacol. 2024 Mar 11;15:1371002. doi: 10.3389/fphar.2024.1371002. eCollection 2024. Front Pharmacol. 2024. PMID: 38529189 Free PMC article. Review.
-
An Insight into Research Advances on Herbal and Phytochemical Approaches to the Management of Hepatocellular Carcinoma from January 2020 to July 2024.Anticancer Agents Med Chem. 2025;25(15):1049-1076. doi: 10.2174/0118715206348951241120120918. Anticancer Agents Med Chem. 2025. PMID: 39936410 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
