Transcriptional and Immune Landscape of Cardiac Sarcoidosis
- PMID: 36111531
- PMCID: PMC9514756
- DOI: 10.1161/CIRCRESAHA.121.320449
Transcriptional and Immune Landscape of Cardiac Sarcoidosis
Abstract
Background: Cardiac involvement is an important determinant of mortality among sarcoidosis patients. Although granulomatous inflammation is a hallmark finding in cardiac sarcoidosis, the precise immune cell populations that comprise the granuloma remain unresolved. Furthermore, it is unclear how the cellular and transcriptomic landscape of cardiac sarcoidosis differs from other inflammatory heart diseases.
Methods: We leveraged spatial transcriptomics (GeoMx digital spatial profiler) and single-nucleus RNA sequencing to elucidate the cellular and transcriptional landscape of cardiac sarcoidosis. Using GeoMX digital spatial profiler technology, we compared the transcriptomal profile of CD68+ rich immune cell infiltrates in human cardiac sarcoidosis, giant cell myocarditis, and lymphocytic myocarditis. We performed single-nucleus RNA sequencing of human cardiac sarcoidosis to identify immune cell types and examined their transcriptomic landscape and regulation. Using multichannel immunofluorescence staining, we validated immune cell populations identified by single-nucleus RNA sequencing, determined their spatial relationship, and devised an immunostaining approach to distinguish cardiac sarcoidosis from other inflammatory heart diseases.
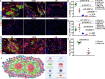
Results: Despite overlapping histological features, spatial transcriptomics identified transcriptional signatures and associated pathways that robustly differentiated cardiac sarcoidosis from giant cell myocarditis and lymphocytic myocarditis. Single-nucleus RNA sequencing revealed the presence of diverse populations of myeloid cells in cardiac sarcoidosis with distinct molecular features. We identified GPNMB (transmembrane glycoprotein NMB) as a novel marker of multinucleated giant cells and predicted that the MITF (microphthalmia-associated transcription factor) family of transcription factors regulated this cell type. We also detected additional macrophage populations in cardiac sarcoidosis including HLA-DR (human leukocyte antigen-DR)+ macrophages, SYTL3 (synaptotagmin-like protein 3)+ macrophages and CD163+ resident macrophages. HLA-DR+ macrophages were found immediately adjacent to GPMMB+ giant cells, a distinct feature compared with other inflammatory cardiac diseases. SYTL3+ macrophages were located scattered throughout the granuloma and CD163+ macrophages, CD1c+ dendritic cells, nonclassical monocytes, and T cells were located at the periphery and outside of the granuloma. Finally, we demonstrate mTOR (mammalian target of rapamycin) pathway activation is associated with proliferation and is selectively found in HLA-DR+ and SYLT3+ macrophages.
Conclusions: In this study, we identified diverse populations of immune cells with distinct molecular signatures that comprise the sarcoid granuloma. These findings provide new insights into the pathology of cardiac sarcoidosis and highlight opportunities to improve diagnostic testing.
Keywords: giant cells; granuloma; inflammation; macrophages; myocarditis; sarcoidosis.
Figures





Comment in
-
Mapping Human Cardiac Sarcoidosis by Single Nuclear and Spatial Profiling.Circ Res. 2022 Sep 30;131(8):670-672. doi: 10.1161/CIRCRESAHA.122.321778. Epub 2022 Sep 29. Circ Res. 2022. PMID: 36173822 No abstract available.
References
-
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–2165. doi: 10.1056/NEJMra071714 - PubMed
-
- Yatsynovich Y, Dittoe N, Petrov M, Maroz N. Cardiac sarcoidosis: a review of contemporary challenges in diagnosis and treatment. Am J Med Sci. 2018;355:113–125. doi: 10.1016/j.amjms.2017.08.009 - PubMed
-
- Ardehali H, Howard DL, Hariri A, Qasim A, Hare JM, Baughman KL, Kasper EK. A positive endomyocardial biopsy result for sarcoid is associated with poor prognosis in patients with initially unexplained cardiomyopathy. Am Heart J. 2005;150:459–463. doi: 10.1016/j.ahj.2004.10.006 - PubMed
-
- Matsui Y, Iwai K, Tachibana T, Fruie T, Shigematsu N, Izumi T, Homma AH, Mikami R, Hongo O, Hiraga Y., et al. . Clinicopathological study of fatal myocardial sarcoidosis. Ann N Y Acad Sci. 1976;278:455–469. doi: 10.1111/j.1749-6632.1976.tb47058.x - PubMed
-
- Perry A, Vuitch F. Causes of death in patients with sarcoidosis. A morphologic study of 38 autopsies with clinicopathologic correlations. Arch Pathol Lab Med. 1995;119:167–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

