CircRNA.0007127 triggers apoptosis through the miR-513a-5p/CASP8 axis in K-562 cells
- PMID: 36111570
- PMCID: PMC9483609
- DOI: 10.1631/jzus.B2200048
CircRNA.0007127 triggers apoptosis through the miR-513a-5p/CASP8 axis in K-562 cells
Abstract
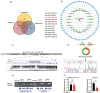
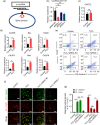
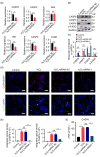
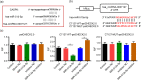
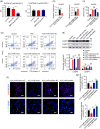
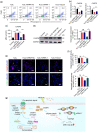
BACKGROUND: Circular RNAs (circRNAs) are covalently closed single-stranded RNAs with multiple biological functions. CircRNA.0007127 is derived from the carbon catabolite repression 4-negative on TATA-less (CCR4-NOT) complex subunit 2 (CNOT2), which was found to regulate tumor cell apoptosis through caspase pathway. METHODS: Potential circRNA.0007127 target microRNAs (miRNAs) were analyzed by miRanda, TargetScan, and RNAhybrid software, and the miRNAs with binding sites for apoptosis-related genes were screened. The roles of circRNA.0007127 and its downstream target, microRNA (miR)-513a-5p, were validated by quantitative real-time polymerase chain reaction (qPCR), flow cytometry, mitochondrial membrane potential, immunofluorescence, western blot, and caspase-8 (CASP8) protein activity in vitro in H2O2-induced K-562 cells. The circRNA.0007127‒miR-513a-5p and CASP8‒miR-513a-5p interactions were verified by luciferase reporter assays. RESULTS: Silencing circRNA.0007127 decreased cell apoptosis by inhibiting CASP8 pathway activation in K-562 cells. Compared with the control group, the expression of CASP8 was reduced by 50% and the 43-kD fragment of CASP8 protein was significantly reduced (P≤0.05). The luciferase reporting assay showed that circRNA.0007127 combined with miR-513a-5p or CASP8, with extremely significant differences (P≤0.001). The overexpression of miR-513a-5p inhibited the gene expression level of CASP8 in a human myeloid leukemia cell model (75% change) and the level of a 43-kD fragment of CASP8 protein (P≤0.01). The rescue experiment showed that cotransfection with circRNA.0007127 small-interfering RNA (siRNA) and the miR-513a-5p inhibitor increased CASP8 gene expression and the apoptosis rate, suggesting that the miR-513a-5p inhibitor is a circRNA.0007127 siRNA antagonist. CONCLUSIONS: CircRNA.0007127 regulates K-562 cell apoptosis through the miR-513a-5p/CASP8 axis, which can serve as a novel powerful molecular target for K-562 cells.
Keywords: Apoptosis; Caspase-8 (CASP8); CircRNA.0007127; K-562 cells; miR-513a-5p.
Figures
Similar articles
-
[Deleted in lymphocytic leukemia 1 promoted proliferation and apoptosis of nephroblastoma cells through regulating miR-513a-5p and RANBP2 pathway].Zhonghua Zhong Liu Za Zhi. 2020 Oct 23;42(10):849-855. doi: 10.3760/cma.j.cn112152-20200311-00194. Zhonghua Zhong Liu Za Zhi. 2020. PMID: 33113626 Chinese.
-
Overexpression of circRNA_100290 promotes the progression of laryngeal squamous cell carcinoma through the miR-136-5p/RAP2C axis.Biomed Pharmacother. 2020 May;125:109874. doi: 10.1016/j.biopha.2020.109874. Epub 2020 Jan 31. Biomed Pharmacother. 2020. PMID: 32014687
-
The CircRNA-ACAP2/Hsa-miR-21-5p/ Tiam1 Regulatory Feedback Circuit Affects the Proliferation, Migration, and Invasion of Colon Cancer SW480 Cells.Cell Physiol Biochem. 2018;49(4):1539-1550. doi: 10.1159/000493457. Epub 2018 Sep 13. Cell Physiol Biochem. 2018. PMID: 30212824
-
Protective role of circRNA CCND1 in ulcerative colitis via miR-142-5p/NCOA3 axis.BMC Gastroenterol. 2023 Jan 19;23(1):18. doi: 10.1186/s12876-023-02641-6. BMC Gastroenterol. 2023. PMID: 36658474 Free PMC article.
-
MicroRNA-513a-5p mediates TNF-α and LPS induced apoptosis via downregulation of X-linked inhibitor of apoptotic protein in endothelial cells.Biochimie. 2012 Jun;94(6):1431-6. doi: 10.1016/j.biochi.2012.03.023. Epub 2012 Apr 5. Biochimie. 2012. PMID: 22504158
Cited by
-
Non-coding RNAs regulating mitochondrial function in cardiovascular diseases.J Mol Med (Berl). 2023 May;101(5):501-526. doi: 10.1007/s00109-023-02305-8. Epub 2023 Apr 4. J Mol Med (Berl). 2023. PMID: 37014377 Review.
-
Circ_PIAS1 Promotes the Apoptosis of ALV-J Infected DF1 Cells by Up-Regulating miR-183.Genes (Basel). 2023 Jun 14;14(6):1260. doi: 10.3390/genes14061260. Genes (Basel). 2023. PMID: 37372440 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

