Studying actin-induced cell shape changes using Giant Unilamellar Vesicles and reconstituted actin networks
- PMID: 36111807
- PMCID: PMC9704537
- DOI: 10.1042/BST20220900
Studying actin-induced cell shape changes using Giant Unilamellar Vesicles and reconstituted actin networks
Abstract
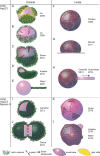
Cell shape changes that are fuelled by the dynamics of the actomyosin cytoskeleton control cellular processes such as motility and division. However, the mechanisms of interplay between cell membranes and actomyosin are complicated to decipher in the complex environment of the cytoplasm. Using biomimetic systems offers an alternative approach to studying cell shape changes in assays with controlled biochemical composition. Biomimetic systems allow quantitative experiments that can help to build physical models describing the processes of cell shape changes. This article reviews works in which actin networks are reconstructed inside or outside cell-sized Giant Unilamellar Vesicles (GUVs), which are models of cell membranes. We show how various actin networks affect the shape and mechanics of GUVs and how some cell shape changes can be reproduced in vitro using these minimal systems.
Keywords: actin; biomimetism; cytoskeleton; membranes.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

