Boron bridging of rhamnogalacturonan-II in Rosa and arabidopsis cell cultures occurs mainly in the endo-membrane system and continues at a reduced rate after secretion
- PMID: 36112021
- PMCID: PMC9670748
- DOI: 10.1093/aob/mcac119
Boron bridging of rhamnogalacturonan-II in Rosa and arabidopsis cell cultures occurs mainly in the endo-membrane system and continues at a reduced rate after secretion
Abstract
Background and aims: Rhamnogalacturonan-II (RG-II) is a domain of primary cell-wall pectin. Pairs of RG-II domains are covalently cross-linked via borate diester bridges, necessary for normal cell growth. Interpreting the precise mechanism and roles of boron bridging is difficult because there are conflicting hypotheses as to whether bridging occurs mainly within the Golgi system, concurrently with secretion or within the cell wall. We therefore explored the kinetics of RG-II bridging.
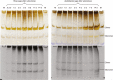
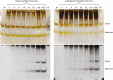
Methods: Cell-suspension cultures of Rosa and arabidopsis were pulse-radiolabelled with [14C]glucose, then the boron bridging status of newly synthesized [14C]RG-II domains was tracked by polyacrylamide gel electrophoresis of endo-polygalacturonase digests.
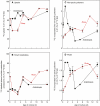
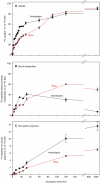
Key results: Optimal culture ages for 14C-labelling were ~5 and ~1 d in Rosa and arabidopsis respectively. De-novo [14C]polysaccharide production occurred for the first ~90 min; thereafter the radiolabelled molecules were tracked as they 'aged' in the wall. Monomeric and (boron-bridged) dimeric [14C]RG-II domains appeared simultaneously, both being detectable within 4 min of [14C]glucose feeding, i.e. well before the secretion of newly synthesized [14C]polysaccharides into the apoplast at ~15-20 min. The [14C]dimer : [14C]monomer ratio of RG-II remained approximately constant from 4 to 120 min, indicating that boron bridging was occurring within the Golgi system during polysaccharide biosynthesis. However, [14C]dimers increased slightly over the following 15 h, indicating that limited boron bridging was continuing after secretion.
Conclusions: The results show where in the cell (and thus when in the 'career' of an RG-II domain) boron bridging occurs, helping to define the possible biological roles of RG-II dimerization and the probable localization of boron-donating glycoproteins or glycolipids.
Keywords: Arabidopsis thaliana; Rosa sp. (‘Paul’s Scarlet’); Boron bridges; borate diesters; cell-suspension cultures; cell-wall polysaccharides; pectin; polyacrylamide gel electrophoresis; radiolabelling; rhamnogalacturonan-II.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Annals of Botany Company.
Figures


Similar articles
-
An Arabidopsis thaliana arabinogalactan-protein (AGP31) and several cationic AGP fragments catalyse the boron bridging of rhamnogalacturonan-II.Biochem J. 2022 Sep 30;479(18):1967-1984. doi: 10.1042/BCJ20220340. Biochem J. 2022. PMID: 36062804 Free PMC article.
-
Boron bridging of rhamnogalacturonan-II, monitored by gel electrophoresis, occurs during polysaccharide synthesis and secretion but not post-secretion.Plant J. 2014 Feb;77(4):534-46. doi: 10.1111/tpj.12403. Epub 2014 Jan 24. Plant J. 2014. PMID: 24320597 Free PMC article.
-
Rhamnogalacturonan-II cross-linking of plant pectins via boron bridges occurs during polysaccharide synthesis and/or secretion.Plant Signal Behav. 2014;9(3):e28169. doi: 10.4161/psb.28169. Epub 2014 Mar 6. Plant Signal Behav. 2014. PMID: 24603547 Free PMC article.
-
Arabinogalactan-Proteins as Boron-Acting Enzymes, Cross-Linking the Rhamnogalacturonan-II Domains of Pectin.Plants (Basel). 2023 Nov 21;12(23):3921. doi: 10.3390/plants12233921. Plants (Basel). 2023. PMID: 38068557 Free PMC article. Review.
-
Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide.Annu Rev Plant Biol. 2004;55:109-39. doi: 10.1146/annurev.arplant.55.031903.141750. Annu Rev Plant Biol. 2004. PMID: 15377216 Review.
Cited by
-
Activity of Organoboron Compounds against Biofilm-Forming Pathogens.Antibiotics (Basel). 2024 Sep 29;13(10):929. doi: 10.3390/antibiotics13100929. Antibiotics (Basel). 2024. PMID: 39452196 Free PMC article. Review.
-
What Can Boron Deficiency Symptoms Tell Us about Its Function and Regulation?Plants (Basel). 2023 Feb 9;12(4):777. doi: 10.3390/plants12040777. Plants (Basel). 2023. PMID: 36840125 Free PMC article. Review.
-
An Arabidopsis thaliana arabinogalactan-protein (AGP31) and several cationic AGP fragments catalyse the boron bridging of rhamnogalacturonan-II.Biochem J. 2022 Sep 30;479(18):1967-1984. doi: 10.1042/BCJ20220340. Biochem J. 2022. PMID: 36062804 Free PMC article.
-
Reduced RG-II pectin dimerization disrupts differential growth by attenuating hormonal regulation.Sci Adv. 2025 Feb 14;11(7):eads0760. doi: 10.1126/sciadv.ads0760. Epub 2025 Feb 12. Sci Adv. 2025. PMID: 39937898 Free PMC article.
-
The plant cell wall-dynamic, strong, and adaptable-is a natural shapeshifter.Plant Cell. 2024 May 1;36(5):1257-1311. doi: 10.1093/plcell/koad325. Plant Cell. 2024. PMID: 38301734 Free PMC article. Review.
References
-
- Carpita NC, Montezinos D, Sabularse D, Delmer DP.. 1979. Determination of the pore size of cell walls of living plants. Science 205: 1144–1147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

