Delaying testicular sperm extraction in 47,XXY Klinefelter patients does not impair the sperm retrieval rate, and AMH levels are higher when TESE is positive
- PMID: 36112034
- PMCID: PMC9627253
- DOI: 10.1093/humrep/deac203
Delaying testicular sperm extraction in 47,XXY Klinefelter patients does not impair the sperm retrieval rate, and AMH levels are higher when TESE is positive
Abstract
Study question: Should testicular sperm extraction (TESE) in non-mosaic 47,XXY Klinefelter syndrome (KS) patients be performed soon after puberty or could it be delayed until adulthood?
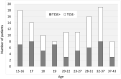
Summary answer: The difference in sperm retrieval rate (SRR) in TESE was not significant between the 'Young' (15-22 years old) cohort and the 'Adult' (23-43 years old) cohort of non-mosaic KS patients recruited prospectively in parallel.
What is known already: Several studies have tried to define predictive factors for TESE outcome in non-mosaic KS patients, with very heterogeneous results. Some authors have found that age was a pejorative factor and recommended performing TESE soon after puberty. To date, no predictive factors have been unanimously recognized to guide clinicians in deciding to perform TESE in azoospermic KS patients.
Study design, size, duration: Two cohorts (Young: 15-22 years old; Adult: 23-43 years old) were included prospectively in parallel. A total of 157 non-mosaic 47,XXY KS patients were included between 2010 and 2020 in the reproductive medicine department of the University Hospital of Lyon, France. However 31 patients gave up before TESE, four had cryptozoospermia and three did not have a valid hormone assessment; these were excluded from this study.
Participants/materials, setting, methods: Data for 119 patients (61 Young and 58 Adult) were analyzed. All of these patients had clinical, hormonal and seminal evaluation before conventional TESE (c-TESE).
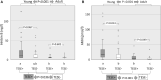
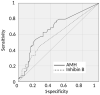
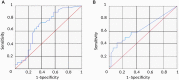
Main results and the role of chance: The global SRR was 45.4%. SRRs were not significantly different between the two age groups: Young SRR=49.2%, Adult SRR = 41.4%; P = 0.393. Anti-Müllerian hormone (AMH) and inhibin B were significantly higher in the Young group (AMH: P = 0.001, Inhibin B: P < 0.001), and also higher in patients with a positive TESE than in those with a negative TESE (AMH: P = 0.001, Inhibin B: P = 0.036). The other factors did not differ between age groups or according to TESE outcome. AMH had a better predictive value than inhibin B. SRRs were significantly higher in the upper quartile of AMH plasma levels than in the lower quartile (or in cases with AMH plasma level below the quantification limit): 67.7% versus 28.9% in the whole population (P = 0.001), 60% versus 20% in the Young group (P = 0.025) and 71.4% versus 33.3% in the Adult group (P = 0.018).
Limitations, reasons for caution: c-TESE was performed in the whole study; we cannot rule out the possibility of different results if microsurgical TESE had been performed. Because of the limited sensitivity of inhibin B and AMH assays, a large number of patients had values lower than the quantification limits, preventing the definition a threshold below which negative TESE can be predicted.
Wider implications of the findings: In contrast to some studies, age did not appear as a pejorative factor when comparing patients 15-22 and 23-44 years of age. Improved accuracy of inhibin B and AMH assays in the future might still allow discrimination of patients with persistent foci of spermatogenesis and guide clinician decision-making and patient information.
Study funding/competing interest(s): The study was supported by a grant from the French Ministry of Health D50621 (Programme Hospitalier de Recherche Clinical Régional 2008). The authors have no conflicts of interest to disclose.
Trial registration number: NCT01918280.
Keywords: Klinefelter syndrome; anti-Müllerian hormone; fertility preservation; male infertility; sperm retrieval.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Figures

Comment in
-
Male Infertility.J Urol. 2023 Jul;210(1):204-206. doi: 10.1097/JU.0000000000003472. Epub 2023 Apr 24. J Urol. 2023. PMID: 37092719 No abstract available.
Similar articles
-
Sperm recovery and ICSI outcomes in Klinefelter syndrome: a systematic review and meta-analysis.Hum Reprod Update. 2017 May 1;23(3):265-275. doi: 10.1093/humupd/dmx008. Hum Reprod Update. 2017. PMID: 28379559
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
Cited by
-
Anti-Müllerian hormone predicts positive sperm retrieval in men with idiopathic non-obstructive azoospermia-findings from a multi-centric cross-sectional study.Hum Reprod. 2023 Aug 1;38(8):1464-1472. doi: 10.1093/humrep/dead125. Hum Reprod. 2023. PMID: 37322566 Free PMC article.
-
Serum Lipocalin-2 Levels as a Biomarker in Pre- and Post-Pubertal Klinefelter Syndrome Patients: A Pilot Study.Int J Mol Sci. 2024 Feb 12;25(4):2214. doi: 10.3390/ijms25042214. Int J Mol Sci. 2024. PMID: 38396890 Free PMC article.
References
-
- Aboukhshaba A, Punjani N, Doukakis S, Schlegel PN.. Anti‐Müllerian hormone level as a predictor of sperm retrieval with microdissection testicular sperm extraction in nonobstructive azoospermia. Andrologia 2021;53:e14220. - PubMed
-
- Aksglaede L, Christiansen P, Sørensen K, Boas M, Linneberg A, Main KM, Andersson A-M, Skakkebaek NE, Juul A.. Serum concentrations of anti-Müllerian hormone (AMH) in 95 patients with Klinefelter syndrome with or without cryptorchidism: AMH concentrations in Klinefelter syndrome, cryptorchidism. Acta Paediatr 2011;100:839–845. - PubMed
-
- Aksglaede L, Sørensen K, Boas M, Mouritsen A, Hagen CP, Jensen RB, Petersen JH, Linneberg A, Andersson A-M, Main KM. et al. Changes in anti-Müllerian hormone (AMH) throughout the life span: a population-based study of 1027 healthy males from birth (cord blood) to the age of 69 years. J Clin Endocrinol Metab 2010;95:5357–5364. - PubMed
-
- Aksglaede L, Wikström AM, Rajpert-De Meyts E, Dunkel L, Skakkebaek NE, Juul A.. Natural history of seminiferous tubule degeneration in Klinefelter syndrome. Hum Reprod Update 2006;12:39–48. - PubMed
-
- Bakircioglu ME, Erden HF, Kaplancan T, Ciray N, Bener F, Bahceci M.. Aging may adversely affect testicular sperm recovery in patients with Klinefelter syndrome. Urology 2006;68:1082–1086. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

