Varicella-zoster virus in actively spreading segmental vitiligo skin: Pathological, immunochemical, and ultrastructural findings (a first and preliminary study)
- PMID: 36112095
- PMCID: PMC10092484
- DOI: 10.1111/pcmr.13064
Varicella-zoster virus in actively spreading segmental vitiligo skin: Pathological, immunochemical, and ultrastructural findings (a first and preliminary study)
Abstract
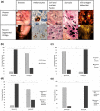
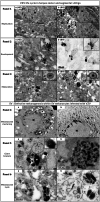
Segmental vitiligo (SV) is a unilateral subtype of vitiligo which is clinically characterized by a cutaneous depigmentation and histologically by a melanocyte loss from the epidermis and hair follicle reservoirs. To date, its pathogenesis remains a mystery. In many cases, this skin depigmentation shares several clinical features and dysfunctions with herpes zoster (HZ). So, for the first time, we examined whether any nucleus and cell fusion associated with a positive immunolabelling of varicella-zoster virus (VZV) and VZV mature virions could be found in SV skin samples as in herpes zoster (HZ). A total of 40 SV samples were used for histological and immunochemical studies. Control samples were obtained from three HZ, and 10 generalized vitiligo lesions. For ultrastructural study, three recent SV and one HZ as controls were recruited. Here, we report that nuclear fusion in epidermal cells were statistically associated with recent SV (p < .001), whereas syncytia formation was associated with long-lasting SV (p = .001). A positive detection of VZV antigen was statistically associated in the epidermis with recent SV and in the dermis with long-lasting SV (p = .001). Finally, the discovery of mature virions in 3/3 recent SV samples provides additional arguments for our viral hypothesis.
Keywords: autonomic nervous system; melanosome transport; nucleus fusion; segmental vitiligo; syncytium; ultrastructural study; varicella-zoster virus; viral cytopathic changes.
© 2022 The Authors. Pigment Cell & Melanoma Research published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Comment in
-
Revisiting the role of varicella zoster virus in segmental vitiligo.Pigment Cell Melanoma Res. 2023 Sep;36(5):439-440. doi: 10.1111/pcmr.13105. Epub 2023 Jun 15. Pigment Cell Melanoma Res. 2023. PMID: 37323109 No abstract available.
References
-
- Arnozan, X. , & Lenoir, L. (1922). Vitiligo avec troubles nerveux sympathiques: l’ origine sympathique du vitiligo. Bulletin de la Société Française de Dermatologie et de Syphiligraphie, 12, 124–140.
-
- Attili, V. R. , & Attili, S. K. (2015). Anatomical segmentations in cell forms of vitiligo: A new dimension of etiopathogenesis. Indian Journal of Dermatology, Venereology and Leprology, 82(4), 379–388. - PubMed
-
- Barra, D. C. , & Seabra, M. C. (2004). The melanosomes as a model to study organelle motility in mammals. Pigment Cell Research, 17(2), 111–118. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

