Pediatric Drug Safety Surveillance: A 10-Year Analysis of Adverse Drug Reaction Reporting Data in Calabria, Southern Italy
- PMID: 36112324
- PMCID: PMC9483327
- DOI: 10.1007/s40264-022-01232-w
Pediatric Drug Safety Surveillance: A 10-Year Analysis of Adverse Drug Reaction Reporting Data in Calabria, Southern Italy
Abstract
Introduction: The paucity of pediatric clinical trials has led to many medicines frequently prescribed to children without a license for use in pediatrics, resulting in an increased risk of adverse drug reactions. Pharmacovigilance databases remain, among others, a valuable tool for evaluating pediatric drug safety in the real-life setting.
Objective: We aimed to characterize pediatric adverse drug reactions reported in the Italian Pharmacovigilance database coming from the Calabria region (Southern Italy) over 10 years.
Methods: All Individual Case Safety Reports (ICSRs) concerning individuals aged under 18 years were extracted from 2010 to 2019. Duplicate and vaccine ICSRs were excluded. The remaining ICSRs were analyzed with respect to patients' demographic data, suspected drugs, and category of adverse drug reactions across different age groups.
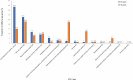
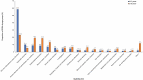
Results: Among 6529 selected ICSRs, 395 pediatric ICSRs corresponding to 556 adverse drug reactions were analyzed. From 2010 to 2015, an increasing number of ICSRs were observed, but the reporting rate decreased after 2015. The highest proportion of ICSRs concerned children and adolescents. Around 52% of ICSRs involved boys: a trend observed in all age groups excluding newborns. Sixty ICSRs were serious and among them, 75% required hospitalization mainly in children and adolescents. Most of the ICSRs were issued by physicians (64.1%), followed by other healthcare professionals (22.5%) and pharmacists (9.9%). Anti-infective agents for systemic use and skin disorders were, respectively, the most frequently reported drug group and adverse drug reaction category.
Conclusions: This study provides an overview of adverse drug reactions reported in the pediatric population of the Calabria region and emphasizes the need for strengthening the surveillance in specific age subgroups and on given drugs in relation to their pattern of use.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare that they have no potential conflicts of interest that might be relevant to the content of this article.
Figures
References
-
- Lindkvist J, Airaksinen M, Kaukonen AM, Klaukka T, Hoppu K. Evolution of paediatric off-label use after new significant medicines become available for adults: a study on triptans in Finnish children 1994–2007. Br J Clin Pharmacol. 2011;71:929–935. doi: 10.1111/j.1365-2125.2010.03881.x. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

