Fabrication Strategies Towards Hydrogels for Biomedical Application: Chemical and Mechanical Insights
- PMID: 36112345
- PMCID: PMC9828515
- DOI: 10.1002/asia.202200797
Fabrication Strategies Towards Hydrogels for Biomedical Application: Chemical and Mechanical Insights
Abstract
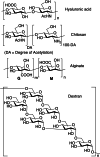
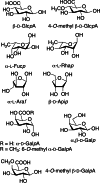
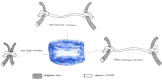
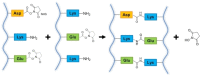
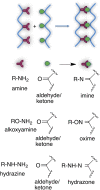
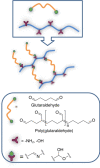
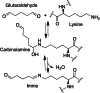
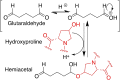
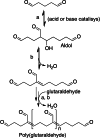
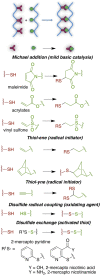
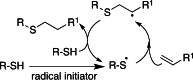
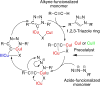
This review aims at giving selected chemical and mechanical insights on design criteria that should be taken into account in hydrogel production for biomedical applications. Particular emphasis will be given to the chemical aspects involved in hydrogel design: macromer chemical composition, cross-linking strategies and chemistry towards "conventional" and smart/stimuli responsive hydrogels. Mechanical properties of hydrogels in view of regenerative medicine applications will also be considered.
Keywords: cross-linking; dynamic reactions; hydrogels; smart hydrogels; stimuli responsiveness.
© 2022 The Authors. Chemistry - An Asian Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


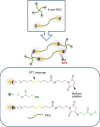
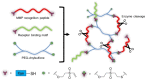


Similar articles
-
Biomedical applications of stimuli-responsive "smart" interpenetrating polymer network hydrogels.Mater Today Bio. 2024 Feb 10;25:100998. doi: 10.1016/j.mtbio.2024.100998. eCollection 2024 Apr. Mater Today Bio. 2024. PMID: 38390342 Free PMC article. Review.
-
Click chemistry-based biopolymeric hydrogels for regenerative medicine.Biomed Mater. 2021 Mar 16;16(2):022003. doi: 10.1088/1748-605X/abc0b3. Biomed Mater. 2021. PMID: 33049725
-
A Review of the Mechanism, Properties, and Applications of Hydrogels Prepared by Enzymatic Cross-linking.J Agric Food Chem. 2023 Jul 12;71(27):10238-10249. doi: 10.1021/acs.jafc.3c01162. Epub 2023 Jun 30. J Agric Food Chem. 2023. PMID: 37390351 Review.
-
A review of gradient stiffness hydrogels used in tissue engineering and regenerative medicine.J Biomed Mater Res A. 2017 Jun;105(6):1799-1812. doi: 10.1002/jbm.a.36034. Epub 2017 Apr 3. J Biomed Mater Res A. 2017. PMID: 28187512 Review.
-
A review of recent advances in biomedical applications of smart cellulose-based hydrogels.Int J Biol Macromol. 2023 Dec 31;253(Pt 6):127149. doi: 10.1016/j.ijbiomac.2023.127149. Epub 2023 Sep 29. Int J Biol Macromol. 2023. PMID: 37778583 Review.
Cited by
-
Hydrogel-based approaches to target hypersensitivity mechanisms underlying autoimmune disease.Adv Drug Deliv Rev. 2024 Sep;212:115395. doi: 10.1016/j.addr.2024.115395. Epub 2024 Jul 14. Adv Drug Deliv Rev. 2024. PMID: 39004347 Free PMC article. Review.
-
A review: Carrier-based hydrogels containing bioactive molecules and stem cells for ischemic stroke therapy.Bioact Mater. 2025 Mar 5;49:39-62. doi: 10.1016/j.bioactmat.2025.01.014. eCollection 2025 Jul. Bioact Mater. 2025. PMID: 40124600 Free PMC article. Review.
-
Medical Applications and Cellular Mechanisms of Action of Carboxymethyl Chitosan Hydrogels.Molecules. 2024 Sep 13;29(18):4360. doi: 10.3390/molecules29184360. Molecules. 2024. PMID: 39339355 Free PMC article. Review.
-
Stimuli-responsive hydrogels: smart state of-the-art platforms for cardiac tissue engineering.Front Bioeng Biotechnol. 2023 Jun 28;11:1174075. doi: 10.3389/fbioe.2023.1174075. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37449088 Free PMC article. Review.
-
Multifunctional DNA-Collagen Biomaterials: Developmental Advances and Biomedical Applications.ACS Biomater Sci Eng. 2025 Mar 10;11(3):1253-1268. doi: 10.1021/acsbiomaterials.4c01475. Epub 2025 Jan 27. ACS Biomater Sci Eng. 2025. PMID: 39869382 Free PMC article. Review.
References
-
- Tanaka T., Fillmore D. J., J. Chem. Phys. 1979, 70, 1214–1218.
-
- Wichterle O., Lím D., Nature 1960, 185, 117–118.
-
- Gaharwar A. K., Singh I., Khademhosseini A., Nat. Rev. Mater. 2020, 5, 686–705.
-
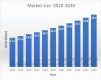
- A. R. and Consulting, “Hydrogel Market Size to reach USD 38,150 Million by 2030 Exclusive Report by Acumen Research and Consulting,” can be found under https://www.globenewswire.com/en/news-release/2022/07/18/2480743/0/en/Hy..., 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

