Autoantibodies against type I IFNs in patients with critical influenza pneumonia
- PMID: 36112363
- PMCID: PMC9485705
- DOI: 10.1084/jem.20220514
Autoantibodies against type I IFNs in patients with critical influenza pneumonia
Abstract
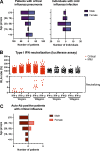
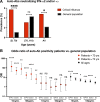
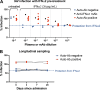
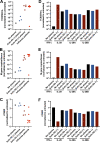
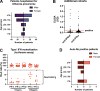
Autoantibodies neutralizing type I interferons (IFNs) can underlie critical COVID-19 pneumonia and yellow fever vaccine disease. We report here on 13 patients harboring autoantibodies neutralizing IFN-α2 alone (five patients) or with IFN-ω (eight patients) from a cohort of 279 patients (4.7%) aged 6-73 yr with critical influenza pneumonia. Nine and four patients had antibodies neutralizing high and low concentrations, respectively, of IFN-α2, and six and two patients had antibodies neutralizing high and low concentrations, respectively, of IFN-ω. The patients' autoantibodies increased influenza A virus replication in both A549 cells and reconstituted human airway epithelia. The prevalence of these antibodies was significantly higher than that in the general population for patients <70 yr of age (5.7 vs. 1.1%, P = 2.2 × 10-5), but not >70 yr of age (3.1 vs. 4.4%, P = 0.68). The risk of critical influenza was highest in patients with antibodies neutralizing high concentrations of both IFN-α2 and IFN-ω (OR = 11.7, P = 1.3 × 10-5), especially those <70 yr old (OR = 139.9, P = 3.1 × 10-10). We also identified 10 patients in additional influenza patient cohorts. Autoantibodies neutralizing type I IFNs account for ∼5% of cases of life-threatening influenza pneumonia in patients <70 yr old.
© 2022 Zhang et al.
Conflict of interest statement
Disclosures: A. Pizzorno reported a patent to WO2016/146836 licensed (Signia Therapeutics), a patent to WO2017/174593 licensed (Signia Therapeutics), and a patent to WO2019/224489 licensed (Signia Therapeutics); and is the co-founder of Signia Therapeutics SAS. N. Le Corre reported personal fees from SINOVAC outside the submitted work. P. Retamar-Gentil reported personal fees from Merck outside the submitted work. I. Meyts reported grants from CSL-Behring outside the submitted work. E. Andreakos reported grants from Janssen Pharmaceuticals during the conduct of the study. J. Wauters reported grants and personal fees from Pfizer and Gilead outside the submitted work. L. Vanderbeke reported grants from Research Foundation Flanders and non-financial support from Pfizer outside the submitted work. S. Feys reported grants from Pfizer outside the submitted work. J. Casalegno reported “other” from Pfizer and grants from Sanofi outside the submitted work. M. Rosa-Calatrava reported a patent to WO2016/146836 licensed (Signia Therapeutics), a patent to WO2017/174593 licensed (Signia Therapeutics), and a patent to WO2019/224489 licensed (Signia Therapeutics); and is the co-founder of Signia Therapeutics SAS. S. Trouillet-Assant reported non-financial support from BioMérieux outside the submitted work. A. Garcia-Sastre reported “other” from Vivaldi Biosciences, Pagoda, Contrafect, Vaxalto, Accurius, Curelab oncology, and Curelab veterinary; personal fees from Avimex, 7Hills, Esperovax, Pfizer, Farmak, Applied Biological Laboratories, Paratus, Pharmamar, Pfizer, and Synairgen; grants from Pfizer, Pharmamar, Blade Therapeutics, Avimex, Accurius, Dynavax, Kenall Manufacturing, ImmunityBio, Nanocomposix, Merck, Model Medicines, Atea Pharma, Shenwa Biosciences, Johnson & Johnson, 7 Hills, Hexamer, N-fold LLC, and Applied Biological Laboratories outside the submitted work; in addition, A. Garcia-Sastre had a patent for influenza virus vaccines and uses thereof issued; and invited speaker in meeting events organized by Seqirus, Janssen, Abbott, and Astrazeneca. J. Casanova reported a patent to PCT/US2021/042741 pending. No other disclosures were reported.
Figures

References
-
- Abers, M.S., Rosen L.B., Delmonte O.M., Shaw E., Bastard P., Imberti L., Quaresima V., Biondi A., Bonfanti P., Castagnoli R., et al. . 2021. Neutralizing type-I interferon autoantibodies are associated with delayed viral clearance and intensive care unit admission in patients with COVID-19. Immunol. Cell Biol. 99:917–921. 10.1111/imcb.12495 - DOI - PMC - PubMed
-
- Abolhassani, H., Landegren N., Bastard P., Materna M., Modaresi M., Du L., Aranda-Guillen M., Sardh F., Zuo F., Zhang P., et al. . 2022. Inherited IFNAR1 deficiency in a child with both critical COVID-19 pneumonia and multisystem inflammatory syndrome. J. Clin. Immunol. 42:471–48310.1007/s10875-022-01215-7 - DOI - PMC - PubMed
-
- Acosta-Ampudia, Y., Monsalve D.M., Rojas M., Rodriguez Y., Gallo J.E., Salazar-Uribe J.C., Santander M.J., Cala M.P., Zapata W., Zapata M.I., et al. . 2021. COVID-19 convalescent plasma composition and immunological effects in severe patients. J. Autoimmu. 118:102598. 10.1016/j.jaut.2021.102598 - DOI - PMC - PubMed
-
- Akbil, B., Meyer T., Stubbemann P., Thibeault C., Staudacher O., Niemeyer D., Jansen J., Mühlemann B., Doehn J., Tabeling C., et al. ; Pa-COVID study Group . 2022. Early and Rapid Identification of COVID-19 Patients with Neutralizing Type I Interferon Auto-antibodies. J. Clin. Immunol. 10.1007/s10875-022-01252-2 - DOI - PMC - PubMed
-
- Asano, T., Boisson B., Onodi F., Matuozzo D., Moncada-Velez M., Maglorius Renkilaraj M.R.L., Zhang P., Meertens L., Bolze A., Materna M., et al. . 2021. X-linked recessive TLR7 deficiency in ∼1% of men under 60 years old with life-threatening COVID-19. Sci. Immunol. 6:eabl4348. 10.1126/sciimmunol.abl4348 - DOI - PMC - PubMed

