A Bivalent Omicron-Containing Booster Vaccine against Covid-19
- PMID: 36112399
- PMCID: PMC9511634
- DOI: 10.1056/NEJMoa2208343
A Bivalent Omicron-Containing Booster Vaccine against Covid-19
Abstract
Background: The safety and immunogenicity of the bivalent omicron-containing mRNA-1273.214 booster vaccine are not known.
Methods: In this ongoing, phase 2-3 study, we compared the 50-μg bivalent vaccine mRNA-1273.214 (25 μg each of ancestral Wuhan-Hu-1 and omicron B.1.1.529 [BA.1] spike messenger RNAs) with the previously authorized 50-μg mRNA-1273 booster. We administered mRNA-1273.214 or mRNA-1273 as a second booster in adults who had previously received a two-dose (100-μg) primary series and first booster (50-μg) dose of mRNA-1273 (≥3 months earlier). The primary objectives were to assess the safety, reactogenicity, and immunogenicity of mRNA-1273.214 at 28 days after the booster dose.
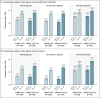
Results: Interim results are presented. Sequential groups of participants received 50 μg of mRNA-1273.214 (437 participants) or mRNA-1273 (377 participants) as a second booster dose. The median time between the first and second boosters was similar for mRNA-1273.214 (136 days) and mRNA-1273 (134 days). In participants with no previous severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, the geometric mean titers of neutralizing antibodies against the omicron BA.1 variant were 2372.4 (95% confidence interval [CI], 2070.6 to 2718.2) after receipt of the mRNA-1273.214 booster and 1473.5 (95% CI, 1270.8 to 1708.4) after receipt of the mRNA-1273 booster. In addition, 50-μg mRNA-1273.214 and 50-μg mRNA-1273 elicited geometric mean titers of 727.4 (95% CI, 632.8 to 836.1) and 492.1 (95% CI, 431.1 to 561.9), respectively, against omicron BA.4 and BA.5 (BA.4/5), and the mRNA-1273.214 booster also elicited higher binding antibody responses against multiple other variants (alpha, beta, gamma, and delta) than the mRNA-1273 booster. Safety and reactogenicity were similar with the two booster vaccines. Vaccine effectiveness was not assessed in this study; in an exploratory analysis, SARS-CoV-2 infection occurred in 11 participants after the mRNA-1273.214 booster and in 9 participants after the mRNA-1273 booster.
Conclusions: The bivalent omicron-containing vaccine mRNA-1273.214 elicited neutralizing antibody responses against omicron that were superior to those with mRNA-1273, without evident safety concerns. (Funded by Moderna; ClinicalTrials.gov number, NCT04927065.).
Copyright © 2022 Massachusetts Medical Society.
Figures


References
-
- CoVariants. Overview of variants in countries. 2021. (https://covariants.org/per-country).
-
- CoVariants. Overview of variants/mutations. 2022. (https://covariants.org/variants).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
