A Probody T Cell-Engaging Bispecific Antibody Targeting EGFR and CD3 Inhibits Colon Cancer Growth with Limited Toxicity
- PMID: 36112781
- PMCID: PMC9664135
- DOI: 10.1158/0008-5472.CAN-21-2483
A Probody T Cell-Engaging Bispecific Antibody Targeting EGFR and CD3 Inhibits Colon Cancer Growth with Limited Toxicity
Abstract
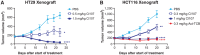
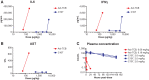
T cell-engaging bispecific antibodies (TCB) are highly potent therapeutics that can recruit and activate cytotoxic T cells to stimulate an antitumor immune response. However, the development of TCBs against solid tumors has been limited by significant on-target toxicity to normal tissues. Probody therapeutics have been developed as a novel class of recombinant, protease-activated antibody prodrugs that are "masked" to reduce antigen binding in healthy tissues but can become conditionally unmasked by proteases that are preferentially active in the tumor microenvironment (TME). Here, we describe the preclinical efficacy and safety of CI107, a Probody TCB targeting EGFR and CD3. In vitro, the protease-activated, unmasked CI107 effectively bound EGFR and CD3 expressed on the surface of cells and induced T-cell activation, cytokine release, and cytotoxicity toward tumor cells. In contrast, dually masked CI107 displayed a >500-fold reduction in antigen binding and >15,000-fold reduction in cytotoxic activity. In vivo, CI107 potently induced dose-dependent tumor regression of established colon cancer xenografts in mice engrafted with human peripheral blood mononuclear cells. Furthermore, the MTD of CI107 in cynomolgus monkeys was more than 60-fold higher than that of the unmasked TCB, and much lower levels of toxicity were observed in animals receiving CI107. Therefore, by localizing activity to the TME and thus limiting toxicity to normal tissues, this Probody TCB demonstrates the potential to expand clinical opportunities for TCBs as effective anticancer therapies for solid tumor indications.
Significance: A conditionally active EGFR-CD3 T cell-engaging Probody therapeutic expands the safety window of bispecific antibodies while maintaining efficacy in preclinical solid tumor settings.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures





References
-
- Trabolsi A, Arumov A, Schatz JH. T cell-activating bispecific antibodies in cancer therapy. J Immunol 2019;203:585–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

