Electrooxidation of Phenol on Polyelectrolyte Modified Carbon Electrodes for Use in Insulin Pump Infusion Sets
- PMID: 36112811
- PMCID: PMC11089874
- DOI: 10.1177/19322968221123083
Electrooxidation of Phenol on Polyelectrolyte Modified Carbon Electrodes for Use in Insulin Pump Infusion Sets
Abstract
Background: Many type 1 diabetes patients using continuous subcutaneous insulin infusion (CSII) suffer from the phenomenon of unexplained hypoglycemia or "site loss." Site loss is hypothesized to be caused by toxic excipients, for example, phenolic compounds within insulin formulations that are used as preservatives and stabilizers. Here, we develop a bioinspired polyelectrolyte-modified carbon electrode for effective electrooxidative removal of phenol from insulin and eventual incorporations into an infusion set of a CSII device.
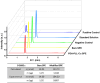
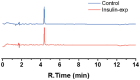
Methods: We modified a carbon screen printed electrode (SPE) with poly-L-lysine (PLL) to avoid passivation due to polyphenol deposition while still removing phenolic compounds from insulin injections. We characterized these electrodes using scanning electron microscopy (SEM) and electrochemical impedance spectroscopy (EIS) and compared their data with data from bare SPEs. Furthermore, we performed electrochemical measurements to determine the extent of passivation, and high-performance liquid chromatography (HPLC) measurements to confirm both the removal of phenol and the integrity of insulin after phenol removal.
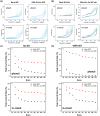
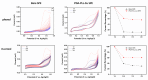
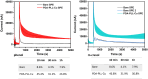
Results: Voltammetry measurements show that electrode passivation due to polyphenol deposition is reduced by a factor of 2X. HPLC measurements confirm a 10x greater removal of phenol by our modified electrodes relative to bare electrodes.
Conclusion: Using bioinspired polyelectrolytes to modify a carbon electrode surface aids in the electrooxidation of phenolic compounds from insulin and is a step toward integration within an infusion set for mitigating site loss.
Keywords: electrooxidation; infusion set; insulin pump; passivation; phenol; phenolic compound.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Similar articles
-
Disposable biosensor based on nanodiamond particles, ionic liquid and poly-l-lysine for determination of phenolic compounds.Anal Biochem. 2024 May;688:115464. doi: 10.1016/j.ab.2024.115464. Epub 2024 Jan 18. Anal Biochem. 2024. PMID: 38244752
-
Continuous subcutaneous insulin infusion vs modern multiple injection regimens in type 1 diabetes: an updated meta-analysis of randomized clinical trials.Acta Diabetol. 2019 Sep;56(9):973-980. doi: 10.1007/s00592-019-01326-5. Epub 2019 Apr 3. Acta Diabetol. 2019. PMID: 30945047
-
Nonmetabolic complications of continuous subcutaneous insulin infusion: a patient survey.Diabetes Technol Ther. 2014 Mar;16(3):145-9. doi: 10.1089/dia.2013.0192. Epub 2013 Nov 1. Diabetes Technol Ther. 2014. PMID: 24180294 Free PMC article.
-
Continuous subcutaneous insulin infusion therapy: A primer on insulin pumps.J Am Pharm Assoc (2003). 2009 Jan-Feb;49(1):e1-13; quiz e14-7. doi: 10.1331/JAphA.2009.08122. J Am Pharm Assoc (2003). 2009. PMID: 19196588 Review.
-
[The use of continuous subcutaneous insulin infusion (CSII) with personal insulin pumps in the treatment of children and adolescents with diabetes type 1].Wiad Lek. 2004;57(5-6):263-6. Wiad Lek. 2004. PMID: 15518073 Review. Polish.
Cited by
-
The DERMIS Study: Methodologies, Results, and Implications for the Future.J Diabetes Sci Technol. 2024 Dec 5:19322968241298005. doi: 10.1177/19322968241298005. Online ahead of print. J Diabetes Sci Technol. 2024. PMID: 39633523 Free PMC article.
References
-
- Priesterroth L, Grammes J, Clauter M, Kubiak T. Diabetes technologies in people with type 1 diabetes mellitus and disordered eating: a systematic review on continuous subcutaneous insulin infusion, continuous glucose monitoring and automated insulin delivery. Diabet Med. 2021;38(7):e14581. doi:10.1111/dme.14581. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

