Development of a Molecular Blood-Based Immune Signature Classifier as Biomarker for Risks Assessment in Lung Cancer Screening
- PMID: 36112827
- PMCID: PMC9627262
- DOI: 10.1158/1055-9965.EPI-22-0689
Development of a Molecular Blood-Based Immune Signature Classifier as Biomarker for Risks Assessment in Lung Cancer Screening
Abstract
Background: Low-dose CT (LDCT) screening trials have shown that lung cancer early detection saves lives. However, a better stratification of the screening population is still needed. In this respect, we generated and prospectively validated a plasma miRNA signature classifier (MSC) able to categorize screening participants according to lung cancer risk. Here, we aimed to deeply characterize the peripheral immune profile and develop a diagnostic immune signature classifier to further implement blood testing in lung cancer screening.
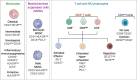
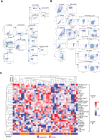
Methods: Peripheral blood mononuclear cell (PBMC) samples collected from 20 patients with LDCT-detected lung cancer and 20 matched cancer-free screening volunteers were analyzed by flow cytometry using multiplex panels characterizing both lymphoid and myeloid immune subsets. Data were validated in PBMC from 40 patients with lung cancer and 40 matched controls and in a lung cancer specificity set including 27 subjects with suspicious lung nodules. A qPCR-based gene expression signature was generated resembling selected immune subsets.
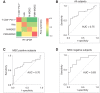
Results: Monocytic myeloid-derived suppressor cell (MDSC), polymorphonuclear MDSC, intermediate monocytes and CD8+PD-1+ T cells distinguished patients with lung cancer from controls with AUCs values of 0.94/0.72/0.88 in the training, validation, and lung cancer specificity set, respectively. AUCs raised up to 1.00/0.84/0.92 in subgroup analysis considering only MSC-negative subjects. A 14-immune genes expression signature distinguished patients from controls with AUC values of 0.76 in the validation set and 0.83 in MSC-negative subjects.
Conclusions: An immune-based classifier can enhance the accuracy of blood testing, thus supporting the contribution of systemic immunity to lung carcinogenesis.
Impact: Implementing LDCT screening trials with minimally invasive blood tests could help reduce unnecessary procedures and optimize cost-effectiveness.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures


Comment in
- 1055-9965. doi: 10.1158/1055-9965.EPI-31-11-HI doi: 10.1158/1055-9965.EPI-31-11-HI
References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7–33. - PubMed
-
- de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, et al. . Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 2020;382:503–13. - PubMed
-
- Jonas DE, Reuland DS, Reddy SM, Nagle M, Clark SD, Weber RP, et al. . Screening for lung cancer with low-dose computed tomography: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2021;325:971–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

