Actin cytoskeleton remodeling primes RIG-I-like receptor activation
- PMID: 36113429
- PMCID: PMC9680832
- DOI: 10.1016/j.cell.2022.08.011
Actin cytoskeleton remodeling primes RIG-I-like receptor activation
Abstract
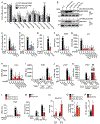
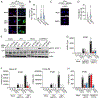
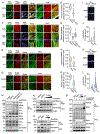
The current dogma of RNA-mediated innate immunity is that sensing of immunostimulatory RNA ligands is sufficient for the activation of intracellular sensors and induction of interferon (IFN) responses. Here, we report that actin cytoskeleton disturbance primes RIG-I-like receptor (RLR) activation. Actin cytoskeleton rearrangement induced by virus infection or commonly used reagents to intracellularly deliver RNA triggers the relocalization of PPP1R12C, a regulatory subunit of the protein phosphatase-1 (PP1), from filamentous actin to cytoplasmic RLRs. This allows dephosphorylation-mediated RLR priming and, together with the RNA agonist, induces effective RLR downstream signaling. Genetic ablation of PPP1R12C impairs antiviral responses and enhances susceptibility to infection with several RNA viruses including SARS-CoV-2, influenza virus, picornavirus, and vesicular stomatitis virus. Our work identifies actin cytoskeleton disturbance as a priming signal for RLR-mediated innate immunity, which may open avenues for antiviral or adjuvant design.
Keywords: MDA5; PP1; RIG-I; actin cytoskeleton; innate immunity; type-I interferon; viral infection.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



Comment in
-
Disturbance of cytoskeleton primes RIG-I-like receptors.Nat Rev Immunol. 2022 Nov;22(11):654-655. doi: 10.1038/s41577-022-00789-y. Nat Rev Immunol. 2022. PMID: 36175502 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

