Immunogenicity and safety of one-dose human papillomavirus vaccine compared with two or three doses in Tanzanian girls (DoRIS): an open-label, randomised, non-inferiority trial
- PMID: 36113531
- PMCID: PMC9638030
- DOI: 10.1016/S2214-109X(22)00309-6
Immunogenicity and safety of one-dose human papillomavirus vaccine compared with two or three doses in Tanzanian girls (DoRIS): an open-label, randomised, non-inferiority trial
Abstract
Background: An estimated 15% of girls aged 9-14 years worldwide have been vaccinated against human papillomavirus (HPV) with the recommended two-dose or three-dose schedules. A one-dose HPV vaccine schedule would be simpler and cheaper to deliver. We report immunogenicity and safety results of different doses of two different HPV vaccines in Tanzanian girls.
Methods: In this open-label, randomised, phase 3, non-inferiority trial, we enrolled healthy schoolgirls aged 9-14 years from Government schools in Mwanza, Tanzania. Eligible participants were randomly assigned to receive one, two, or three doses of either the 2-valent vaccine (Cervarix, GSK Biologicals, Rixensart) or the 9-valent vaccine (Gardasil-9, Sanofi Pasteur MSD, Lyon). The primary outcome was HPV 16 specific or HPV 18 specific seropositivity following one dose compared with two or three doses of the same HPV vaccine 24 months after vaccination. Safety was assessed as solicited adverse events up to 30 days after each dose and unsolicited adverse events up to 24 months after vaccination or to last study visit. The primary outcome was done in the per-protocol population, and safety was analysed in the total vaccinated population. This study was registered in ClinicalTrials.gov, NCT02834637.
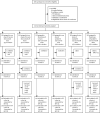
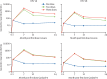
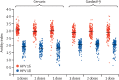
Findings: Between Feb 23, 2017, and Jan 6, 2018, we screened 1002 girls for eligibility. 72 girls were excluded. 930 girls were enrolled and randomly assigned to receive one dose of Cervarix (155 participants), two doses of Cervarix (155 participants), three doses of Cervarix (155 participants), one dose of Gardasil-9 (155 participants), two doses of Gardasil-9 (155 participants), or three doses of Gardasil-9 (155 participants). 922 participants received all scheduled doses within the defined window (three withdrew, one was lost to follow-up, and one died before completion; two received their 6-month doses early, and one received the wrong valent vaccine in error; all 930 participants were included in the total vaccinated cohort). Retention at 24 months was 918 (99%) of 930 participants. In the according-to-protocol cohort, at 24 months, 99% of participants who received one dose of either HPV vaccine were seropositive for HPV 16 IgG antibodies, compared with 100% of participants who received two doses, and 100% of participants who received three doses. This met the prespecified non-inferiority criteria. Anti-HPV 18 seropositivity at 24 months did not meet non-inferiority criteria for one dose compared to two doses or three doses for either vaccine, although more than 98% of girls in all groups had HPV 18 antibodies. 53 serious adverse events (SAEs) were experienced by 42 (4·5%) of 930 girls, the most common of which was hospital admission for malaria. One girl died of malaria. Number of events was similar between groups and no SAEs were considered related to vaccination.
Interpretation: A single dose of the 2-valent or 9-valent HPV vaccine in girls aged 9-14 years induced robust immune responses up to 24 months, suggesting that this reduced dose regimen could be suitable for prevention of HPV infection among girls in the target age group for vaccination.
Funding: UK Department for International Development/UK Medical Research Council/Wellcome Trust Joint Global Health Trials Scheme, The Bill & Melinda Gates Foundation, and the US National Cancer Institute.
Translation: For the KiSwahili translation of the abstract see Supplementary Materials section.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests DW-J reports a grant from GSK Biologicals in 2007 for a previous study on safety and immunogenicity of Cervarix in Tanzania unrelated to this submitted work. All other authors declare no competing interests.
Figures
Comment in
-
HPV vaccines: when one plus one equals three.Lancet Glob Health. 2022 Oct;10(10):e1373-e1374. doi: 10.1016/S2214-109X(22)00373-4. Lancet Glob Health. 2022. PMID: 36113514 No abstract available.
Similar articles
-
Comparing one dose of HPV vaccine in girls aged 9-14 years in Tanzania (DoRIS) with one dose of HPV vaccine in historical cohorts: an immunobridging analysis of a randomised controlled trial.Lancet Glob Health. 2022 Oct;10(10):e1485-e1493. doi: 10.1016/S2214-109X(22)00306-0. Lancet Glob Health. 2022. PMID: 36113532 Free PMC article. Clinical Trial.
-
Comparing one dose of HPV vaccine in girls aged 9-14 years in Tanzania (DoRIS) with one dose in young women aged 15-20 years in Kenya (KEN SHE): an immunobridging analysis of randomised controlled trials.Lancet Glob Health. 2024 Mar;12(3):e491-e499. doi: 10.1016/S2214-109X(23)00586-7. Lancet Glob Health. 2024. PMID: 38365419 Free PMC article. Clinical Trial.
-
Immunogenicity and safety of a new quadrivalent HPV vaccine in girls and boys aged 9-14 years versus an established quadrivalent HPV vaccine in women aged 15-26 years in India: a randomised, active-controlled, multicentre, phase 2/3 trial.Lancet Oncol. 2023 Dec;24(12):1321-1333. doi: 10.1016/S1470-2045(23)00480-1. Epub 2023 Nov 7. Lancet Oncol. 2023. PMID: 37949086 Clinical Trial.
-
Comparison of different human papillomavirus (HPV) vaccine types and dose schedules for prevention of HPV-related disease in females and males.Cochrane Database Syst Rev. 2019 Nov 22;2019(11):CD013479. doi: 10.1002/14651858.CD013479. Cochrane Database Syst Rev. 2019. PMID: 31755549 Free PMC article.
-
Clinical update of the AS04-adjuvanted human papillomavirus-16/18 cervical cancer vaccine, Cervarix.Adv Ther. 2009 Nov;26(11):983-98. doi: 10.1007/s12325-009-0079-5. Epub 2009 Dec 18. Adv Ther. 2009. PMID: 20024678 Review.
Cited by
-
The one-dose schedule opens the door to rapid scale-up of HPV vaccination.BMC Med. 2023 Oct 9;21(1):387. doi: 10.1186/s12916-023-03097-x. BMC Med. 2023. PMID: 37807059 Free PMC article. No abstract available.
-
Immunogenicity and duration of antibodies after vaccination with a two-dose series of the nine-valent human papillomavirus vaccine among Alaska Native children: a prospective cohort study.BMC Infect Dis. 2025 Apr 30;25(1):640. doi: 10.1186/s12879-025-10961-z. BMC Infect Dis. 2025. PMID: 40307681 Free PMC article.
-
Durability of immunogenicity at 5 years after a single dose of human papillomavirus vaccine compared with two doses in Tanzanian girls aged 9-14 years: results of the long-term extension of the DoRIS randomised trial.Lancet Glob Health. 2025 Feb;13(2):e319-e328. doi: 10.1016/S2214-109X(24)00477-7. Lancet Glob Health. 2025. PMID: 39890232 Free PMC article. Clinical Trial.
-
Efficacy, effectiveness and immunogenicity of reduced HPV vaccination schedules: A review of available evidence.Can Commun Dis Rep. 2024 Jun 28;50(6):166-178. doi: 10.14745/ccdr.v50i06a01. eCollection 2024 Jun 28. Can Commun Dis Rep. 2024. PMID: 39021378 Free PMC article.
-
Pathways and processes to adopting or switching to a single-dose HPV vaccination schedule in low- and middle-income countries: a qualitative study.PLOS Glob Public Health. 2025 Feb 5;5(2):e0004245. doi: 10.1371/journal.pgph.0004245. eCollection 2025. PLOS Glob Public Health. 2025. PMID: 39908311 Free PMC article.
References
-
- International Agency for Research on Cancer Cervix uteri. 2020. https://gco.iarc.fr/today/data/factsheets/cancers/23-Cervix-uteri-fact-s...
-
- WHO Global Strategy to accelerate the elimination of cervical cancer as a public health problem. https://www.who.int/publications/i/item/9789240014107
-
- Gallagher KE, LaMontagne DS, Watson-Jones D. Status of HPV vaccine introduction and barriers to country uptake. Vaccine. 2018;36:4761–4767. - PubMed
-
- Bruni L, Saura-Lázaro A, Montoliu A, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev Med. 2021;144 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

