Multiple cryoprotectant toxicity model for vitrification solution optimization
- PMID: 36113568
- PMCID: PMC9529850
- DOI: 10.1016/j.cryobiol.2022.09.002
Multiple cryoprotectant toxicity model for vitrification solution optimization
Abstract
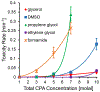
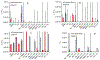
Vitrification is a promising cryopreservation technique for complex specimens such as tissues and organs. However, it is challenging to identify mixtures of cryoprotectants (CPAs) that prevent ice formation without exerting excessive toxicity. In this work, we developed a multi-CPA toxicity model that predicts the toxicity kinetics of mixtures containing five of the most common CPAs used in the field (glycerol, dimethyl sulfoxide (DMSO), propylene glycol, ethylene glycol, and formamide). The model accounts for specific toxicity, non-specific toxicity, and interactions between CPAs. The proposed model shows reasonable agreement with training data for single and binary CPA solutions, as well as ternary CPA solution validation data. Sloppy model analysis was used to examine the model parameters that were most important for predictions, providing clues about mechanisms of toxicity. This analysis revealed that the model terms for non-specific toxicity were particularly important, especially the non-specific toxicity of propylene glycol, as well as model terms for specific toxicity of formamide and interactions between formamide and glycerol. To demonstrate the potential for model-based design of vitrification methods, we paired the multi-CPA toxicity model with a published vitrification/devitrification model to identify vitrifiable CPA mixtures that are predicted to have minimal toxicity. The resulting optimized vitrification solution composition was a mixture of 7.4 molal glycerol, 1.4 molal DMSO, and 2.4 molal formamide. This demonstrates the potential for mathematical optimization of vitrification solution composition and sets the stage for future studies to optimize the complete vitrification process, including CPA mixture composition and CPA addition and removal methods.
Keywords: Cryopreservation; Cryoprotectants; Optimization; Sloppy models; Toxicity; Vitrification.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors have no conflicts of interest.
Figures
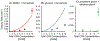
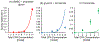


References
-
- Almansoori KA, Prasad V, Forbes JF, Law GK, McGann LE, Elliott JA, Jomha NM, Cryoprotective agent toxicity interactions in human articular chondrocytes, Cryobiology 64 (2012) 185–191. - PubMed
-
- Beier AF, Schulz JC, Dorr D, Katsen-Globa A, Sachinidis A, Hescheler J, Zimmermann H, Effective surface-based cryopreservation of human embryonic stem cells by vitrification, Cryobiology 63 (2011) 175–185. - PubMed
-
- Benson JD, Kearsley AJ, Higgins AZ, Mathematical optimization of procedures for cryoprotectant equilibration using a toxicity cost function, Cryobiology 64 (2012) 144–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

