Modular nanoarray vaccine for SARS-CoV-2
- PMID: 36113829
- PMCID: PMC9468299
- DOI: 10.1016/j.nano.2022.102604
Modular nanoarray vaccine for SARS-CoV-2
Abstract
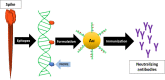
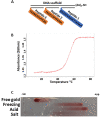
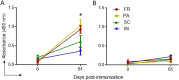
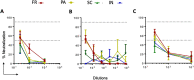
The current vaccine development strategies for the COVID-19 pandemic utilize whole inactive or attenuated viruses, virus-like particles, recombinant proteins, and antigen-coding DNA and mRNA with various delivery strategies. While highly effective, these vaccine development strategies are time-consuming and often do not provide reliable protection for immunocompromised individuals, young children, and pregnant women. Here, we propose a novel modular vaccine platform to address these shortcomings using chemically synthesized peptides identified based on the validated bioinformatic data about the target. The vaccine is based on the rational design of an immunogen containing two defined B-cell epitopes from the spike glycoprotein of SARS-CoV-2 and the universal T-helper epitope PADRE. The epitopes were conjugated to short DNA probes and combined with a complementary scaffold strand, resulting in sequence-specific self-assembly. The immunogens were then formulated by conjugation to gold nanoparticles by three methods or by co-crystallization with epsilon inulin. BALB/C mice were immunized with each formulation, and the IgG immune responses and virus neutralizing titers were compared. The results demonstrate that this assembly is immunogenic and generates neutralizing antibodies against wildtype SARS-CoV-2 and the Delta variant.
Keywords: Epitope; PADRE; Peptide; SARS-CoV-2; Vaccine; Variants of concerns (VOC).
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest Authors declare no competing interests.
Figures


References
-
- Self W.H., Tenforde M.W., Rhoads J.P., et al. Comparative effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions — United States, March–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(38):1337–1343. doi: 10.15585/mmwr.mm7038e1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

