Structure-based discovery of small molecules that disaggregate Alzheimer's disease tissue derived tau fibrils in vitro
- PMID: 36114178
- PMCID: PMC9481533
- DOI: 10.1038/s41467-022-32951-4
Structure-based discovery of small molecules that disaggregate Alzheimer's disease tissue derived tau fibrils in vitro
Abstract
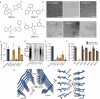
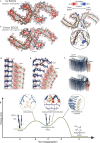
Alzheimer's disease (AD) is the consequence of neuronal death and brain atrophy associated with the aggregation of protein tau into fibrils. Thus disaggregation of tau fibrils could be a therapeutic approach to AD. The small molecule EGCG, abundant in green tea, has long been known to disaggregate tau and other amyloid fibrils, but EGCG has poor drug-like properties, failing to fully penetrate the brain. Here we have cryogenically trapped an intermediate of brain-extracted tau fibrils on the kinetic pathway to EGCG-induced disaggregation and have determined its cryoEM structure. The structure reveals that EGCG molecules stack in polar clefts between the paired helical protofilaments that pathologically define AD. Treating the EGCG binding position as a pharmacophore, we computationally screened thousands of drug-like compounds for compatibility for the pharmacophore, discovering several that experimentally disaggregate brain-derived tau fibrils in vitro. This work suggests the potential of structure-based, small-molecule drug discovery for amyloid diseases.
© 2022. The Author(s).
Conflict of interest statement
D.S.E. is SAB chair and equity holder of ADRx, Inc. All other authors declare no conflicts.
Figures


References
-
- LMTM. ALZFORUM. https://www.alzforum.org/therapeutics/lmtm (2019).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

