Zinc chelator treatment in crush syndrome model mice attenuates ischemia-reperfusion-induced muscle injury due to suppressing of neutrophil infiltration
- PMID: 36114355
- PMCID: PMC9481620
- DOI: 10.1038/s41598-022-19903-0
Zinc chelator treatment in crush syndrome model mice attenuates ischemia-reperfusion-induced muscle injury due to suppressing of neutrophil infiltration
Abstract
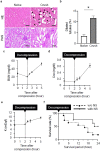
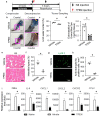
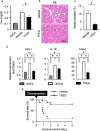
In crush syndrome, massive muscle breakdown resulting from ischemia-reperfusion muscle injury can be a life-threatening condition that requires urgent treatment. Blood reperfusion into the ischemic muscle triggers an immediate inflammatory response, and neutrophils are the first to infiltrate and exacerbate the muscle damage. Since free zinc ion play a critical role in the immune system and the function of neutrophils is impaired by zinc depletion, we hypothesized that the administration of a zinc chelator would be effective for suppressing the inflammatory reaction at the site of ischemia-reperfusion injury and for improving of the pathology of crush syndrome. A crush syndrome model was created by using a rubber tourniquet to compress the bilateral hind limbs of mice at 8 weeks. A zinc chelator N,N,N',N'-tetrakis-(2-pyridylmethyl)-ethylenediamine (TPEN) was administered immediately after reperfusion in order to assess the anti-inflammatory effect of the chelator for neutrophils. Histopathological evaluation showed significantly less muscle breakdown and fewer neutrophil infiltration in TPEN administration group compared with control group. In addition, the expression levels of inflammatory cytokine and chemokine such as IL-6, TNFα, CXCL1, CXCL2, CXCR2, CCL2 in ischemia-reperfusion injured muscle were significantly suppressed with TPEN treatment. Less dilatation of renal tubules in histological evaluation in renal tissue and significantly better survival rate were demonstrated in TPEN treatment for ischemia-reperfusion injury in crush syndrome. The findings of our study suggest that zinc chelators contributed to the resolution of exacerbation of the inflammatory response and attenuation of muscle breakdown in the acute phase after crush syndrome. In addition, our strategy of attenuation of the acute inflammatory reaction by zinc chelators may provide a promising therapeutic strategy not only for crush syndrome, but also for other diseases driven by inflammatory reactions.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- von Colmers F. Uber die durch das Erbeben in Messina. Arch. fur klin. Chir. 1909;90:701–747.
-
- Bywaters EGL, Beall D, Bywaters GL, Knochel P. Crush injuries with impairment by of renal. J. Am. Soc. Nephrol. 1941;1:427–432.

