Autoprocessing and oxyanion loop reorganization upon GC373 and nirmatrelvir binding of monomeric SARS-CoV-2 main protease catalytic domain
- PMID: 36114420
- PMCID: PMC9481597
- DOI: 10.1038/s42003-022-03910-y
Autoprocessing and oxyanion loop reorganization upon GC373 and nirmatrelvir binding of monomeric SARS-CoV-2 main protease catalytic domain
Abstract
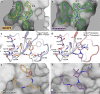
The monomeric catalytic domain (residues 1-199) of SARS-CoV-2 main protease (MPro1-199) fused to 25 amino acids of its flanking nsp4 region mediates its autoprocessing at the nsp4-MPro1-199 junction. We report the catalytic activity and the dissociation constants of MPro1-199 and its analogs with the covalent inhibitors GC373 and nirmatrelvir (NMV), and the estimated monomer-dimer equilibrium constants of these complexes. Mass spectrometry indicates the presence of the accumulated adduct of NMV bound to MProWT and MPro1-199 and not of GC373. A room temperature crystal structure reveals a native-like fold of the catalytic domain with an unwound oxyanion loop (E state). In contrast, the structure of a covalent complex of the catalytic domain-GC373 or NMV shows an oxyanion loop conformation (E* state) resembling the full-length mature dimer. These results suggest that the E-E* equilibrium modulates autoprocessing of the main protease when converting from a monomeric polyprotein precursor to the mature dimer.
© 2022. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Visualizing the Active Site Oxyanion Loop Transition Upon Ensitrelvir Binding and Transient Dimerization of SARS-CoV-2 Main Protease.J Mol Biol. 2024 Jul 1;436(13):168616. doi: 10.1016/j.jmb.2024.168616. Epub 2024 May 16. J Mol Biol. 2024. PMID: 38762033 Free PMC article.
-
Unmasking the Conformational Stability and Inhibitor Binding to SARS-CoV-2 Main Protease Active Site Mutants and Miniprecursor.J Mol Biol. 2022 Dec 30;434(24):167876. doi: 10.1016/j.jmb.2022.167876. Epub 2022 Nov 2. J Mol Biol. 2022. PMID: 36334779 Free PMC article.
-
Insights into the mechanism of SARS-CoV-2 main protease autocatalytic maturation from model precursors.Commun Biol. 2023 Nov 13;6(1):1159. doi: 10.1038/s42003-023-05469-8. Commun Biol. 2023. PMID: 37957287 Free PMC article.
-
Protease targeted COVID-19 drug discovery and its challenges: Insight into viral main protease (Mpro) and papain-like protease (PLpro) inhibitors.Bioorg Med Chem. 2021 Jan 1;29:115860. doi: 10.1016/j.bmc.2020.115860. Epub 2020 Nov 6. Bioorg Med Chem. 2021. PMID: 33191083 Free PMC article. Review.
-
Structural Catalytic Core of the Members of the Superfamily of Acid Proteases.Molecules. 2024 Jul 23;29(15):3451. doi: 10.3390/molecules29153451. Molecules. 2024. PMID: 39124857 Free PMC article. Review.
Cited by
-
Distal protein-protein interactions contribute to nirmatrelvir resistance.Nat Commun. 2025 Feb 1;16(1):1266. doi: 10.1038/s41467-025-56651-x. Nat Commun. 2025. PMID: 39893201 Free PMC article.
-
In vitro enzymatic and cell culture assays for SARS-CoV-2 main protease interaction with ambenonium.Sci Rep. 2025 Mar 27;15(1):10606. doi: 10.1038/s41598-025-94283-9. Sci Rep. 2025. PMID: 40148508 Free PMC article.
-
Characterization of an unusual SARS-CoV-2 main protease natural variant exhibiting resistance to nirmatrelvir and ensitrelvir.Commun Biol. 2025 Jul 17;8(1):1061. doi: 10.1038/s42003-025-08487-w. Commun Biol. 2025. PMID: 40676153 Free PMC article.
-
Visualizing the Active Site Oxyanion Loop Transition Upon Ensitrelvir Binding and Transient Dimerization of SARS-CoV-2 Main Protease.J Mol Biol. 2024 Jul 1;436(13):168616. doi: 10.1016/j.jmb.2024.168616. Epub 2024 May 16. J Mol Biol. 2024. PMID: 38762033 Free PMC article.
-
Assay Development and Validation for Innovative Antiviral Development Targeting the N-Terminal Autoprocessing of SARS-CoV-2 Main Protease Precursors.Viruses. 2024 Jul 29;16(8):1218. doi: 10.3390/v16081218. Viruses. 2024. PMID: 39205192 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

