Protocol: a simple method for biosensor visualization of bacterial quorum sensing and quorum quenching interaction on Medicago roots
- PMID: 36114554
- PMCID: PMC9479286
- DOI: 10.1186/s13007-022-00944-5
Protocol: a simple method for biosensor visualization of bacterial quorum sensing and quorum quenching interaction on Medicago roots
Abstract
Background: Defining interactions of bacteria in the rhizosphere (encompassing the area near and on the plant root) is important to understand how they affect plant health. Some rhizosphere bacteria, including plant growth promoting rhizobacteria (PGPR) engage in the intraspecies communication known as quorum sensing (QS). Many species of Gram-negative bacteria use extracellular autoinducer signal molecules called N-acyl homoserine lactones (AHLs) for QS. Other rhizobacteria species, including PGPRs, can interfere with or disrupt QS through quorum quenching (QQ). Current AHL biosensor assays used for screening and identifying QS and QQ bacteria interactions fail to account for the role of the plant root.
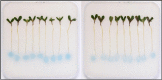
Methods: Medicago spp. seedlings germinated on Lullien agar were transferred to soft-agar plates containing the broad-range AHL biosensor Agrobacterium tumefaciens KYC55 and X-gal substrate. Cultures of QS and QQ bacteria as well as pure AHLs and a QQ enzyme were applied to the plant roots and incubated for 3 days.
Results: We show that this expanded use of an AHL biosensor successfully allowed for visualization of QS/QQ interactions localized at the plant root. KYC55 detected pure AHLs as well as AHLs from live bacteria cultures grown directly on the media. We also showed clear detection of QQ interactions occurring in the presence of the plant root.
Conclusions: Our novel tri-trophic system using an AHL biosensor is useful to study QS interspecies interactions in the rhizosphere.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Arachis hypogaea L. produces mimic and inhibitory quorum sensing like molecules.Antonie Van Leeuwenhoek. 2017 Jul;110(7):891-902. doi: 10.1007/s10482-017-0862-2. Epub 2017 Mar 29. Antonie Van Leeuwenhoek. 2017. PMID: 28357693
-
Novel N-Acyl Homoserine Lactone-Degrading Bacteria Isolated From Penicillin-Contaminated Environments and Their Quorum-Quenching Activities.Front Microbiol. 2019 Mar 14;10:455. doi: 10.3389/fmicb.2019.00455. eCollection 2019. Front Microbiol. 2019. PMID: 30923518 Free PMC article.
-
Linking Short-Chain N-Acyl Homoserine Lactone-Mediated Quorum Sensing and Replant Disease: A Case Study of Rehmannia glutinosa.Front Plant Sci. 2020 Jun 17;11:787. doi: 10.3389/fpls.2020.00787. eCollection 2020. Front Plant Sci. 2020. PMID: 32625222 Free PMC article.
-
Innovative microbial disease biocontrol strategies mediated by quorum quenching and their multifaceted applications: A review.Front Plant Sci. 2023 Jan 12;13:1063393. doi: 10.3389/fpls.2022.1063393. eCollection 2022. Front Plant Sci. 2023. PMID: 36714722 Free PMC article. Review.
-
Quenching of acyl-homoserine lactone-dependent quorum sensing by enzymatic disruption of signal molecules.Acta Biochim Pol. 2009;56(1):1-16. Epub 2009 Mar 17. Acta Biochim Pol. 2009. PMID: 19287806 Review.
References
-
- Gamalero E, Glick BR. Mechanisms used by plant growth-promoting bacteria. In: Maheshwari DK, editor. Bacteria in agrobiology: plant nutrient management. Berlin: Springer; 2011. pp. 17–46.
-
- Kloepper JW, Leong J, Teintze M, Schroth MN. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature. 1980;286:885–886. doi: 10.1038/286885a0. - DOI
-
- Bassler BL, Miller MB. Quorum sensing. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F, editors. The Prokaryotes. Berlin: Springer; 2013. pp. 495–509.
-
- Chernin LS. Quorum-sensing signals as mediators of PGPRs’ beneficial traits. In: Maheshwari DK, editor. Bacteria in agrobiology: plant nutrient management. Berlin: Springer; 2011. pp. 209–236.
LinkOut - more resources
Full Text Sources
Miscellaneous

