Near-infrared diffuse in vivo flow cytometry
- PMID: 36114606
- PMCID: PMC9478904
- DOI: 10.1117/1.JBO.27.9.097002
Near-infrared diffuse in vivo flow cytometry
Abstract
Significance: Diffuse in vivo flow cytometry (DiFC) is an emerging technique for enumerating rare fluorescently labeled circulating cells noninvasively in the bloodstream. Thus far, we have reported red and blue-green versions of DiFC. Use of near-infrared (NIR) fluorescent light would in principle allow use of DiFC in deeper tissues and would be compatible with emerging NIR fluorescence molecular contrast agents.
Aim: We describe the design of a NIR-DiFC instrument and demonstrate its use in optical flow phantoms in vitro and in mice in vivo.
Approach: We developed an improved optical fiber probe design for efficient collection of fluorescence from individual circulating cells and efficient rejection of instrument autofluorescence. We built a NIR-DiFC instrument. We tested this with NIR fluorescent microspheres and cell lines labeled with OTL38 fluorescence contrast agent in a flow phantom model. We also tested NIR-DiFC in nude mice injected intravenously with OTL38-labeled L1210A cells.
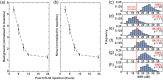
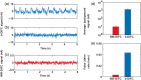
Results: NIR-DiFC allowed detection of circulating tumor cells (CTCs) in flow phantoms with mean signal-to-noise ratios (SNRs) of 19 to 32 dB. In mice, fluorescently labeled CTCs were detectable with mean SNR of 26 dB. NIR-DiFC also exhibited orders significantly lower autofluorescence and false-alarm rates than blue-green DiFC.
Conclusions: NIR-DiFC allows use of emerging NIR contrast agents. Our work could pave the way for future use of NIR-DiFC in humans.
Keywords: contrast agents; diffuse fluorescence; diffuse in vivo flow cytometry; near-infrared light.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

