mSep: investigating physiological and immune-metabolic biomarkers in septic and healthy pregnant women to predict feto-maternal immune health - a prospective observational cohort study protocol
- PMID: 36115679
- PMCID: PMC9486348
- DOI: 10.1136/bmjopen-2022-066382
mSep: investigating physiological and immune-metabolic biomarkers in septic and healthy pregnant women to predict feto-maternal immune health - a prospective observational cohort study protocol
Abstract
Introduction: Maternal sepsis remains a leading cause of death in pregnancy. Physiological adaptations to pregnancy obscure early signs of sepsis and can result in delays in recognition and treatment. Identifying biomarkers that can reliably diagnose sepsis will reduce morbidity and mortality and antibiotic overuse. We have previously identified an immune-metabolic biomarker network comprising three pathways with a >99% accuracy for detecting bacterial neonatal sepsis. In this prospective study, we will describe physiological parameters and novel biomarkers in two cohorts-healthy pregnant women and pregnant women with suspected sepsis-with the aim of mapping pathophysiological drivers and evaluating predictive biomarkers for diagnosing maternal sepsis.
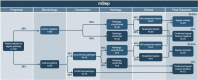
Methods and analysis: Women aged over 18 with an ultrasound-confirmed pregnancy will be recruited to a pilot and two main study cohorts. The pilot will involve blood sample collection from 30 pregnant women undergoing an elective caesarean section. Cohort A will follow 100 healthy pregnant women throughout their pregnancy journey, with collection of blood samples from participants at routine time points in their pregnancy: week 12 'booking', week 28 and during labour. Cohort B will follow 100 pregnant women who present with suspected sepsis in pregnancy or labour and will have at least two blood samples taken during their care pathway. Study blood samples will be collected during routine clinical blood sampling. Detailed medical history and physiological parameters at the time of blood sampling will be recorded, along with the results of routine biochemical tests, including C reactive protein, lactate and white blood cell count. In addition, study blood samples will be processed and analysed for transcriptomic, lipidomic and metabolomic analyses and both qualitative and functional immunophenotyping.
Ethics and dissemination: Ethical approval has been obtained from the Wales Research Ethics Committee 2 (SPON1752-19, 30 October 2019).
Trial registration number: NCT05023954.
Keywords: Immunology; Infection control; OBSTETRICS.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Bauer M E, Bauer S, Rajala B. Maternal physiologic parameters in relationship to systemic inflammatory response syndrome criteria a systematic review and meta-analysis 2014. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials