Digitalized transcranial electrical stimulation: A consensus statement
- PMID: 36115809
- PMCID: PMC10031774
- DOI: 10.1016/j.clinph.2022.08.018
Digitalized transcranial electrical stimulation: A consensus statement
Abstract
Objective: Although relatively costly and non-scalable, non-invasive neuromodulation interventions are treatment alternatives for neuropsychiatric disorders. The recent developments of highly-deployable transcranial electric stimulation (tES) systems, combined with mobile-Health technologies, could be incorporated in digital trials to overcome methodological barriers and increase equity of access. The study aims are to discuss the implementation of tES digital trials by performing a systematic scoping review and strategic process mapping, evaluate methodological aspects of tES digital trial designs, and provide Delphi-based recommendations for implementing digital trials using tES.
Methods: We convened 61 highly-productive specialists and contacted 8 tES companies to assess 71 issues related to tES digitalization readiness, and processes, barriers, advantages, and opportunities for implementing tES digital trials. Delphi-based recommendations (>60% agreement) were provided.
Results: The main strengths/opportunities of tES were: (i) non-pharmacological nature (92% of agreement), safety of these techniques (80%), affordability (88%), and potential scalability (78%). As for weaknesses/threats, we listed insufficient supervision (76%) and unclear regulatory status (69%). Many issues related to methodological biases did not reach consensus. Device appraisal showed moderate digitalization readiness, with high safety and potential for trial implementation, but low connectivity.
Conclusions: Panelists recognized the potential of tES for scalability, generalizability, and leverage of digital trials processes; with no consensus about aspects regarding methodological biases.
Significance: We further propose and discuss a conceptual framework for exploiting shared aspects between mobile-Health tES technologies with digital trials methodology to drive future efforts for digitizing tES trials.
Keywords: Delphi panel; Digital health; Mobile Health; Non-invasive neuromodulation; Systematic review.
Copyright © 2022 International Federation of Clinical Neurophysiology. All rights reserved.
Figures


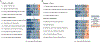
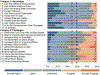

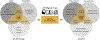
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

