An international working group consensus report for the prioritization of molecular biomarkers for Ewing sarcoma
- PMID: 36115869
- PMCID: PMC9482616
- DOI: 10.1038/s41698-022-00307-2
An international working group consensus report for the prioritization of molecular biomarkers for Ewing sarcoma
Abstract
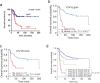
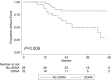
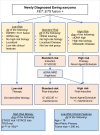
The advent of dose intensified interval compressed therapy has improved event-free survival for patients with localized Ewing sarcoma (EwS) to 78% at 5 years. However, nearly a quarter of patients with localized tumors and 60-80% of patients with metastatic tumors suffer relapse and die of disease. In addition, those who survive are often left with debilitating late effects. Clinical features aside from stage have proven inadequate to meaningfully classify patients for risk-stratified therapy. Therefore, there is a critical need to develop approaches to risk stratify patients with EwS based on molecular features. Over the past decade, new technology has enabled the study of multiple molecular biomarkers in EwS. Preliminary evidence requiring validation supports copy number changes, and loss of function mutations in tumor suppressor genes as biomarkers of outcome in EwS. Initial studies of circulating tumor DNA demonstrated that diagnostic ctDNA burden and ctDNA clearance during induction are also associated with outcome. In addition, fusion partner should be a pre-requisite for enrollment on EwS clinical trials, and the fusion type and structure require further study to determine prognostic impact. These emerging biomarkers represent a new horizon in our understanding of disease risk and will enable future efforts to develop risk-adapted treatment.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Brennan B, et al. Comparison of two chemotherapy regimens in Ewing sarcoma (ES): Overall and subgroup results of the Euro Ewing 2012 randomized trial (EE2012) J. Clin. Oncol. 2020;38:11500–11500. doi: 10.1200/JCO.2020.38.15_suppl.11500. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

