Expression of phosphorylated ribosomal protein S6 in mesothelioma patients - correlation with clinico-pathological characteristics and outcome: results from the European Thoracic Oncology Platform (ETOP) Mesoscape project
- PMID: 36115922
- PMCID: PMC9708564
- DOI: 10.1038/s41379-022-01145-0
Expression of phosphorylated ribosomal protein S6 in mesothelioma patients - correlation with clinico-pathological characteristics and outcome: results from the European Thoracic Oncology Platform (ETOP) Mesoscape project
Erratum in
-
Correction to: Expression of phosphorylated ribosomal protein S6 in mesothelioma patients - correlation with clinico-pathological characteristics and outcome: results from the European Thoracic Oncology Platform (ETOP) Mesoscape project.Mod Pathol. 2022 Dec;35(12):2033. doi: 10.1038/s41379-022-01170-z. Mod Pathol. 2022. PMID: 36253399 Free PMC article. No abstract available.
Abstract
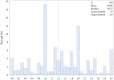
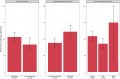
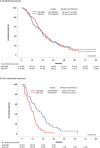
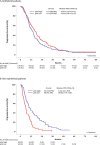
Pleural mesothelioma (PM) is an aggressive malignancy with poor prognosis. Although histology and pathologic stage are important prognostic factors, better prognostic biomarkers are needed. The ribosomal protein S6 is a downstream target of the phosphatidylinositol 3-kinase (PI3K) pathway involved in protein synthesis and cell proliferation. In previous studies, low phosphorylated S6 (pS6) immunoreactivity was significantly correlated with longer progression-free survival (PFS) and overall survival (OS) in PM patients. We aimed to correlate pS6 expression to clinical data in a large multi-centre PM cohort as part of the European Thoracic Oncology Platform (ETOP) Mesoscape project. Tissue Micro Arrays (TMAs) of PM were constructed and expression of pS6 was evaluated by a semi-quantitatively aggregate H-score. Expression results were correlated to patient characteristics as well as OS/PFS. pS6 IHC results of 364 patients from 9 centres, diagnosed between 1999 and 2017 were available. The primary histology of included tumours was epithelioid (70.3%), followed by biphasic (24.2%) and sarcomatoid (5.5%). TMAs included both treatment-naïve and tumour tissue taken after induction chemotherapy. High pS6 expression (181 patients with H-score>1.41) was significantly associated with less complete resection. In the overall cohort, OS/PFS were not significantly different between pS6-low and pS6-high patients. In a subgroup analysis non-epithelioid (biphasic and sarcomatoid) patients with high pS6 expression showed a significantly shorter OS (p < 0.001, 10.7 versus 16.9 months) and PFS (p < 0.001, 6.2 versus 10.8 months). In subgroup analysis, in non-epithelioid PM patients high pS6 expression was associated with significantly shorter OS and PFS. These exploratory findings suggest a clinically relevant PI3K pathway activation in non-epithelioid PM which might lay the foundation for future targeted treatment strategies.
© 2022. The Author(s).
Conflict of interest statement
JHR, MH, ZT, KN, MDP, ST, SGG, LA, EFB, MBK, KM, BW, FBZ, MS, RL, SPF, ES, NM, RK, RAS declared no conflict of interest. EN declares his participation in the following advisoriy boards and lectures: Roche, BMS, MSD, Merck-Serono, Pfizer, Lilly, Amgen, Boehringer-Ingelheim, AstraZeneca, Takeda, Sanofi and Bayer and his research support from: Roche, Pfizer, Merck-Serono and BMS. JA declares: Adv board BMS, Eli-Lilly, MSD, Bayer, Amphera, Roche/Stock owner Amphera/Patent on allogenic tumor lysate, combination immunotherapy. JVDT declares: Adv board and lectures: AstraZeneca, BMS, Eli-Lilly, Johnson&Johnson, MSD, Pfizer, Roche. SP delclares: I have received education grants, provided consultation, attended advisory boards and/or provided lectures for the following organizations, from whom I have received honoraria (all fees to institution): Consultation / Advisory role: AbbVie, Amgen, AstraZeneca, Bayer, Beigene, Biocartis, Boehringer Ingelheim, Bristol-Myers Squibb, Clovis, Daiichi Sankyo, Debiopharm, ecancer, Eli Lilly, Elsevier, Foundation Medicine, Illumina, Imedex, IQVIA, Incyte, Janssen, Medscape, Merck Sharp and Dohme, Merck Serono, Merrimack, Novartis, OncologyEducation, Pharma Mar, Phosplatin Therapeutics, PER, Pfizer, PRIME, Regeneron, RMEI, Roche/Genentech, RTP, Sanofi, Seattle Genetics, Takeda.Talk in a company’s organized public event: AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, ecancer, Eli Lilly, Illumina, Imedex, Medscape, Merck Sharp and Dohme, Novartis, PER, Pfizer, Prime, Roche/Genentech, RTP, Sanofi, Takeda.Receipt of grants/research supports: (Sub)investigator in trials (institutional financial support for clinical trials) sponsored by Amgen, AstraZeneca, Biodesix, Boehringer Ingelheim, Bristol-Myers Squibb, Clovis, GSK, Illumina, Lilly, Merck Sharp and Dohme, Merck Serono, Mirati, Novartis, and Pfizer, Phosplatin Therapeutics, Roche/Genentech. PB declares: Consultancy roel for BMS, Advisory Board for MSD, Beigene, Pfizer, Takeda and Boehringer Ingelheim. Research Grants from AZ and BMS. IO declares: Consultation / Advisory role: AstraZeneca, Bristol-Myers Squibb, MSD Merck Sharp & Dohme AG. Talk in a company’s organized public event: AstraZeneca, Roche. Receipt of grants/research supports: Institutional grants for fellowships from Medtronic, Roche. Proctorship for Intuitive.
Figures

References
-
- Carbone M, Kratzke RA, Testa JR. The pathogenesis of mesothelioma. Sem Oncol 29, 2–17 (2002) - PubMed
-
- Flores RM, Zakowski M, Venkatraman E, Krug L, Rosenzweig K, Dycoco J, et al. Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J Thorac Oncol 2, 957–965 2007) - PubMed

