Aquaporin-4 cerebrospinal fluid levels are higher in neurodegenerative dementia: looking at glymphatic system dysregulation
- PMID: 36115967
- PMCID: PMC9482276
- DOI: 10.1186/s13195-022-01077-6
Aquaporin-4 cerebrospinal fluid levels are higher in neurodegenerative dementia: looking at glymphatic system dysregulation
Abstract
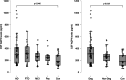
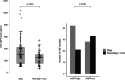
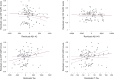
Aquaporin-4 (AQP4) is a channel protein that plays a fundamental role in glymphatic system, a newly described pathway for fluid exchange in the central nervous system, as well as a central figure in a fascinating new theory for the pathophysiology of neurodegenerative diseases such as Alzheimer's disease (AD) and frontotemporal dementia (FTD). In this study, cerebrospinal fluid (CSF) concentration of AQP4, amyloid-β, total tau and P-tau were determined in 103 CSF samples from patients affected by neurodegenerative dementias (AD and FTD) or psychiatric diseases and 21 controls. Significantly higher levels of AQP4 were found in AD and FTD patients compared to subjects not affected by neurodegenerative diseases, and a significant, positive correlation between AQP4 and total tau levels was found. This evidence may pave the way for future studies focused on the role of this channel protein in the clinical assessment of the glymphatic function and degree of neurodegeneration.
Keywords: Alzheimer’s disease; Amyloid-β; Aquaporin 4; Cerebrospinal fluid; Frontotemporal dementia; Glymphatic system.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Ménard J, Zetterberg H, Wisniewski T, de Leon MJ. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015;11(8):457–70. doi: 10.1038/nrneurol.2015.119. - DOI - PMC - PubMed
-
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111. doi: 10.1126/scitranslmed.3003748. - DOI - PMC - PubMed
-
- Mestre H, Hablitz LM, Xavier AL, Feng W, Zou W, Pu T, Monai H, Murlidharan G, Castellanos Rivera RM, Simon MJ, Pike MM, Plá V, Du T, Kress BT, Wang X, Plog BA, Thrane AS, Lundgaard I, Abe Y, Yasui M, Thomas JH, Xiao M, Hirase H, Asokan A, Iliff JJ, Nedergaard M. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife. 2018;18(7):e40070. doi: 10.7554/eLife.40070. - DOI - PMC - PubMed

