Redox imbalance in COVID-19 pathophysiology
- PMID: 36116160
- PMCID: PMC9464257
- DOI: 10.1016/j.redox.2022.102465
Redox imbalance in COVID-19 pathophysiology
Abstract
Background: The pathophysiologic significance of redox imbalance is unquestionable as numerous reports and topic reviews indicate alterations in redox parameters during corona virus disease 2019 (COVID-19). However, a more comprehensive understanding of redox-related parameters in the context of COVID-19-mediated inflammation and pathophysiology is required.
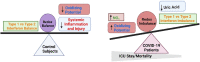
Methods: COVID-19 subjects (n = 64) and control subjects (n = 19) were enrolled, and blood was drawn within 72 h of diagnosis. Serum multiplex assays and peripheral blood mRNA sequencing was performed. Oxidant/free radical (electron paramagnetic resonance (EPR) spectroscopy, nitrite-nitrate assay) and antioxidant (ferrous reducing ability of serum assay and high-performance liquid chromatography) were performed. Multivariate analyses were performed to evaluate potential of indicated parameters to predict clinical outcome.
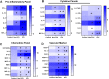
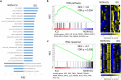
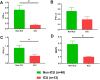
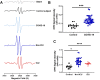
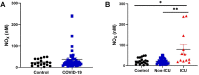
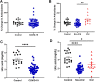
Results: Significantly greater levels of multiple inflammatory and vascular markers were quantified in the subjects admitted to the ICU compared to non-ICU subjects. Gene set enrichment analyses indicated significant enhancement of oxidant related pathways and biochemical assays confirmed a significant increase in free radical production and uric acid reduction in COVID-19 subjects. Multivariate analyses confirmed a positive association between serum levels of VCAM-1, ICAM-1 and a negative association between the abundance of one electron oxidants (detected by ascorbate radical formation) and mortality in COVID subjects while IL-17c and TSLP levels predicted need for intensive care in COVID-19 subjects.
Conclusion: Herein we demonstrate a significant redox imbalance during COVID-19 infection affirming the potential for manipulation of oxidative stress pathways as a new therapeutic strategy COVID-19. However, further work is requisite for detailed identification of oxidants (O2•-, H2O2 and/or circulating transition metals such as Fe or Cu) contributing to this imbalance to avoid the repetition of failures using non-specific antioxidant supplementation.
Keywords: COVID-19; EPR; Electron paramagnetic resonance; Redox imbalance; SARS-CoV-2; Transcriptomics; Uric acid.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., Xing F., Liu J., Yip C.C., Poon R.W., Tsoi H.W., Lo S.K., Chan K.H., Poon V.K., Chan W.M., Ip J.D., Cai J.P., Cheng V.C., Chen H., Hui C.K., Yuen K.Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. - PMC - PubMed
-
- W.H. Organization . 2021. WHO Coronavirus (COVID-19) Dashboard.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

