Mucoadhesive carriers for oral drug delivery
- PMID: 36116580
- PMCID: PMC9960552
- DOI: 10.1016/j.jconrel.2022.09.024
Mucoadhesive carriers for oral drug delivery
Abstract
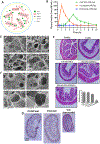
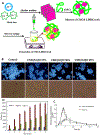
Among the various dosage forms, oral medicine has extensive benefits including ease of administration and patients' compliance, over injectable, suppositories, ocular and nasal. Despite of extensive demand and emerging advantages, over 50% of therapeutic molecules are not available in oral form due to their physicochemical properties. More importantly, most of the biologics, proteins, peptide, and large molecular drugs are mostly available in injectable form. Conventional oral drug delivery system has limitation such as degradation and lack of stability within stomach due to presence of highly acidic gastric fluid, hinders their therapeutic efficacy and demand more frequent and higher dosing. Hence, formulation for controlled, sustained, and targeted drug delivery, need to be designed with feasibility to target the specific region of gastrointestinal (GI) tract such as stomach, small intestine, intestine lymphatic, and colon is challenging. Among various oral delivery approaches, mucoadhesive vehicles are promising and has potential for improving oral drug retention and controlled absorption to treat local diseases within the GI tract, as well systemic diseases. This review provides the overview about the challenges and opportunities to design mucoadhesive formulation for oral delivery of therapeutics in a way to target the specific region of the GI tract. Finally, we have concluded with future perspective and potential of mucoadhesive formulations for oral local and systemic delivery.
Keywords: Gastric cancer; Inflammatory bowel disease; Mucoadhesive polymer; Oral delivery; Oral medicine.
Copyright © 2022 Elsevier B.V. All rights reserved.
Figures
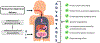
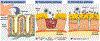








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

