The DEXA-SEPSIS study protocol: a phase II randomized double-blinded controlled trial of the effect of early dexamethasone in high-risk sepsis patients
- PMID: 36116775
- PMCID: PMC9561197
- DOI: 10.15441/ceem.22.276
The DEXA-SEPSIS study protocol: a phase II randomized double-blinded controlled trial of the effect of early dexamethasone in high-risk sepsis patients
Abstract
Objective: Steroids are used in cases of sepsis, especially in patients experiencing septic shock. However, clinical trials to date have reported contradictory results. Different patient endotypes and variations in the type and dose of steroid may be at fault for this discrepancy, and further investigation is warranted. In this paper, we propose a new DEXA-SEPSIS study design.
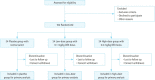
Methods: We plan to conduct a multicenter, double-blinded randomized pilot study (DEXA-SEPSIS) investigating the feasibility and safety of early use of dexamethasone in sepsis. Participants will be high-risk septic patients presenting to the emergency department with a systolic blood pressure of <90 mmHg or serum lactate level of >2 mmol/L. Participants will be randomized to the following three groups: control, 0.1 mg/kg of dexamethasone, or 0.2 mg/kg of dexamethasone per day for 1 to 2 days. The primary outcome will be 28-day mortality. Secondary outcomes will include time to septic shock, shock reversal, additional steroid administration, number of ventilator-free days, use of continuous renal-replacement therapy, length of stay in the intensive care unit and/or hospital, delta Sequential Organ Failure Assessment score on days 3 and 7, superinfection, gastrointestinal bleeding, hypernatremia, and hyperglycemia.
Discussion: The DEXA-SEPSIS study will provide insight regarding the feasibility and safety of early use of dexamethasone in high-risk sepsis. The results could provide data to design a future phase III study.
Trial registration: ClinicalTrials.gov Identifier: NCT05136560.
Keywords: Dexamethasone; Glucocorticoids; Sepsis.
Conflict of interest statement
Kyuseok Kim serves as an editor of the
Figures
References
-
- Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing sepsis as a global health priority: a WHO resolution. N Engl J Med. 2017;377:414–7. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical