Osteogenesis in human periodontal ligament stem cell sheets is enhanced by the protease-activated receptor 1 (PAR1) in vivo
- PMID: 36117187
- PMCID: PMC9482923
- DOI: 10.1038/s41598-022-19520-x
Osteogenesis in human periodontal ligament stem cell sheets is enhanced by the protease-activated receptor 1 (PAR1) in vivo
Abstract
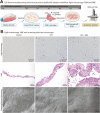
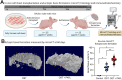
Human periodontal ligament stem cells (PDLSCs) have been studied as a promising strategy in regenerative approaches. The protease-activated receptor 1 (PAR1) plays a key role in osteogenesis and has been shown to induce osteogenesis and increase bone formation in PDLSCs. However, little is known about its effects when activated in PDLSCs as a cell sheet construct and how it would impact bone formation as a graft in vivo. Here, PDLSCs were obtained from 3 patients. Groups were divided into control, osteogenic medium and osteogenic medium + PAR1 activation by TFLLR-NH2 peptide. Cell phenotype was determined by flow cytometry and immunofluorescence. Calcium deposition was quantified by Alizarin Red Staining. Cell sheet microstructure was analyzed through light, scanning electron microscopy and histology and transplanted to Balb/c nude mice. Immunohistochemistry for bone sialoprotein (BSP), integrin β1 and collagen type 1 and histological stains (H&E, Van Giesson, Masson's Trichrome and Von Kossa) were performed on the ex-vivo mineralized tissue after 60 days of implantation in vivo. Ectopic bone formation was evaluated through micro-CT. PAR1 activation increased calcium deposition in vitro as well as BSP, collagen type 1 and integrin β1 protein expression and higher ectopic bone formation (micro-CT) in vivo.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

