Clinical performance of SARS-CoV-2 detection on the cobas Liat using water gargle samples
- PMID: 36117531
- PMCID: PMC9468300
- DOI: 10.1016/j.jcvp.2022.100108
Clinical performance of SARS-CoV-2 detection on the cobas Liat using water gargle samples
Abstract
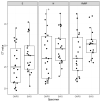
Spring water gargle (SWG) is a suitable, non-invasive, alternative specimen for SARS-CoV-2 detection by RT-PCR. This study sought to evaluate the performance of the cobas Liat point-of-care system for the detection of SARS-CoV-2 in SWG samples. SWG samples and standard oral and nasopharyngeal swab (ONPS) were collected simultaneously from participants in a COVID-19 screening clinic, in November and December 2020. Both sample types were analyzed in parallel on the cobas Liat platform and with the Seegene Allplex 2019-nCoV assay. Among the 110 participants, 53% had compatible symptoms and 71% had a contact with a confirmed COVID-19 case. Only two (1.8%) individuals had neither symptoms nor contact. Amongst 110 paired samples, 25 (23%) were positive for SARS-CoV-2 on the cobas Liat for a least one sample type, with a kappa coefficient of 0.92. Agreement between the cobas Liat platform and the Seegene assay was also excellent (kappa coefficient values of 0.94 and 0.95). Two SWG samples failed to provide a positive result when their ONPS pair was positive, but their cycle threshold (Ct) values were >35 on the Seegene assay, reflecting a low viral load. Overall, the performance of the cobas Liat platform is excellent for the detection of SARS-CoV-2 in SWG samples in a high pre-test probability population.
Keywords: COVID-19; Gargle; SARS-CoV-2; cobas Liat.
© 2022 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Tsang H.F., Leung W.M.S., Chan L.W.C., Cho W.C.S., Wong S.C.C. Performance comparison of the Cobas® Liat® and Cepheid® GeneXpert® systems on SARS-CoV-2 detection in nasopharyngeal swab and posterior oropharyngeal saliva. Expert Rev. Mol. Diagn. 2021;21(5):515–518. doi: 10.1080/14737159.2021.1919513. - DOI - PMC - PubMed
-
- Blackall D., Moreno R., Jin J., Plotinsky R., Dworkin R., Oethinger M. Performance Characteristics of the Roche Diagnostics cobas Liat PCR System as a COVID-19 Screening Tool for Hospital Admissions in a Regional Health Care Delivery System. J. Clin. Microbiol. 2021;59(10) doi: 10.1128/JCM.01278-21. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
