Therapeutic properties of multi-cannabinoid treatment strategies for Alzheimer's disease
- PMID: 36117622
- PMCID: PMC9479694
- DOI: 10.3389/fnins.2022.962922
Therapeutic properties of multi-cannabinoid treatment strategies for Alzheimer's disease
Abstract
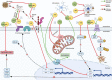
Alzheimer's disease (AD) is a debilitating neurodegenerative disease characterized by declining cognition and behavioral impairment, and hallmarked by extracellular amyloid-β plaques, intracellular neurofibrillary tangles (NFT), oxidative stress, neuroinflammation, and neurodegeneration. There is currently no cure for AD and approved treatments do not halt or slow disease progression, highlighting the need for novel therapeutic strategies. Importantly, the endocannabinoid system (ECS) is affected in AD. Phytocannabinoids, including cannabidiol (CBD) and Δ9-tetrahydrocannabinol (THC), interact with the ECS, have anti-inflammatory, antioxidant, and neuroprotective properties, can ameliorate amyloid-β and NFT-related pathologies, and promote neurogenesis. Thus, in recent years, purified CBD and THC have been evaluated for their therapeutic potential. CBD reversed and prevented the development of cognitive deficits in AD rodent models, and low-dose THC improved cognition in aging mice. Importantly, CBD, THC, and other phytochemicals present in Cannabis sativa interact with each other in a synergistic fashion (the "entourage effect") and have greater therapeutic potential when administered together, rather than individually. Thus, treatment of AD using a multi-cannabinoid strategy (such as whole plant cannabis extracts or particular CBD:THC combinations) may be more efficacious compared to cannabinoid isolate treatment strategies. Here, we review the current evidence for the validity of using multi-cannabinoid formulations for AD therapy. We discuss that such treatment strategies appear valid for AD therapy but further investigations, particularly clinical studies, are required to determine optimal dose and ratio of cannabinoids for superior effectiveness and limiting potential side effects. Furthermore, it is pertinent that future in vivo and clinical investigations consider sex effects.
Keywords: Alzheimer’s disease; cannabidiol (CBD); cannabis extract; cannabis therapeutics; delta-9-tetrahydrocannabinol (THC); dementia; endocannabinod system.
Copyright © 2022 Coles, Steiner-Lim and Karl.
Conflict of interest statement
GZS and TK have received funding from medicinal cannabis companies to conduct research on medicinal cannabis products, outside of this study. The funders were not involved in the manuscript design, synthesis, critical analysis, interpretation of data, the writing of this article or the decision to submit it for publication. The remaining author declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
In vivo Evidence for Therapeutic Properties of Cannabidiol (CBD) for Alzheimer's Disease.Front Pharmacol. 2017 Feb 3;8:20. doi: 10.3389/fphar.2017.00020. eCollection 2017. Front Pharmacol. 2017. PMID: 28217094 Free PMC article. Review.
-
Current Aspects of the Endocannabinoid System and Targeted THC and CBD Phytocannabinoids as Potential Therapeutics for Parkinson's and Alzheimer's Diseases: a Review.Mol Neurobiol. 2020 Nov;57(11):4878-4890. doi: 10.1007/s12035-020-02054-6. Epub 2020 Aug 19. Mol Neurobiol. 2020. PMID: 32813239 Free PMC article. Review.
-
Cannabis-based medicine reduces multiple pathological processes in AβPP/PS1 mice.J Alzheimers Dis. 2015;43(3):977-91. doi: 10.3233/JAD-141014. J Alzheimers Dis. 2015. PMID: 25125475
-
A Critical Review of the Role of the Cannabinoid Compounds Δ9-Tetrahydrocannabinol (Δ9-THC) and Cannabidiol (CBD) and their Combination in Multiple Sclerosis Treatment.Molecules. 2020 Oct 25;25(21):4930. doi: 10.3390/molecules25214930. Molecules. 2020. PMID: 33113776 Free PMC article. Review.
-
A randomised controlled trial of vaporised Δ9-tetrahydrocannabinol and cannabidiol alone and in combination in frequent and infrequent cannabis users: acute intoxication effects.Eur Arch Psychiatry Clin Neurosci. 2019 Feb;269(1):17-35. doi: 10.1007/s00406-019-00978-2. Epub 2019 Jan 19. Eur Arch Psychiatry Clin Neurosci. 2019. PMID: 30661105 Clinical Trial.
Cited by
-
Alzheimer's disease, aging, and cannabidiol treatment: a promising path to promote brain health and delay aging.Mol Biol Rep. 2024 Jan 16;51(1):121. doi: 10.1007/s11033-023-09162-1. Mol Biol Rep. 2024. PMID: 38227160 Review.
-
Cannabinoids and healthy ageing: the potential for extending healthspan and lifespan in preclinical models with an emphasis on Caenorhabditis elegans.Geroscience. 2024 Dec;46(6):5643-5661. doi: 10.1007/s11357-024-01162-8. Epub 2024 May 2. Geroscience. 2024. PMID: 38696056 Free PMC article. Review.
-
A combination of Δ9-tetrahydrocannabinol and cannabidiol modulates glutamate dynamics in the hippocampus of an animal model of Alzheimer's disease.Neurotherapeutics. 2024 Sep;21(5):e00439. doi: 10.1016/j.neurot.2024.e00439. Epub 2024 Sep 3. Neurotherapeutics. 2024. PMID: 39232876 Free PMC article.
-
The Cannabinoids, CBDA and THCA, Rescue Memory Deficits and Reduce Amyloid-Beta and Tau Pathology in an Alzheimer's Disease-like Mouse Model.Int J Mol Sci. 2023 Apr 6;24(7):6827. doi: 10.3390/ijms24076827. Int J Mol Sci. 2023. PMID: 37047798 Free PMC article.
-
Multiple Roles of Apolipoprotein E4 in Oxidative Lipid Metabolism and Ferroptosis During the Pathogenesis of Alzheimer's Disease.J Mol Neurosci. 2024 Jul 3;74(3):62. doi: 10.1007/s12031-024-02224-4. J Mol Neurosci. 2024. PMID: 38958788 Free PMC article. Review.
References
-
- Aizpurua-Olaizola O., Elezgarai I., Rico-Barrio I., Zarandona I., Etxebarria N., Usobiaga A. (2017). Targeting the endocannabinoid system: future therapeutic strategies. Drug Discovery Today 22 105–110. - PubMed
-
- Alzheimer’s Association (2021). 2021 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 17 327–406. - PubMed
-
- Arkell T. R., Lintzeris N., Kevin R. C., Ramaekers J. G., Vandrey R., Irwin C., et al. (2019). Cannabidiol (CBD) content in vaporized cannabis does not prevent tetrahydrocannabinol (THC)-induced impairment of driving and cognition. Psychopharmacology 236 2713–2724. 10.1007/s00213-019-05246-8 - DOI - PMC - PubMed
-
- Arkell T. R., McCartney D., McGregor I. S. (2021). Medical cannabis and driving. Australian J. General Practice 50 357–362. - PubMed
-
- Aso E., Andres-Benito P., Carmona M., Maldonado R., Ferrer I. (2016a). Cannabinoid receptor 2 participates in amyloid-β processing in a mouse model of Alzheimer’s disease but plays a minor role in the therapeutic properties of a cannabis-based medicine. J. Alzheimer’s Dis. 51 489–500. 10.3233/JAD-150913 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

