Pcdh11x controls target specification of mossy fiber sprouting
- PMID: 36117624
- PMCID: PMC9475199
- DOI: 10.3389/fnins.2022.888362
Pcdh11x controls target specification of mossy fiber sprouting
Abstract
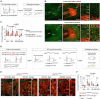
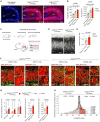
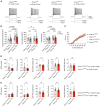
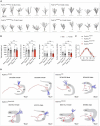
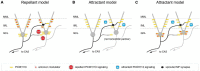
Circuit formation is a defining characteristic of the developing brain. However, multiple lines of evidence suggest that circuit formation can also take place in adults, the mechanisms of which remain poorly understood. Here, we investigated the epilepsy-associated mossy fiber (MF) sprouting in the adult hippocampus and asked which cell surface molecules define its target specificity. Using single-cell RNAseq data, we found lack and expression of Pcdh11x in non-sprouting and sprouting neurons respectively. Subsequently, we used CRISPR/Cas9 genome editing to disrupt the Pcdh11x gene and characterized its consequences on sprouting. Although MF sprouting still developed, its target specificity was altered. New synapses were frequently formed on granule cell somata in addition to dendrites. Our findings shed light onto a key molecular determinant of target specificity in MF sprouting and contribute to understanding the molecular mechanism of adult brain rewiring.
Keywords: Pcdh11x; axonal rewiring; cell adhesion molecule; granule cell; mossy fiber sprouting; protocadherin; synaptic adhesion molecule; target specificity.
Copyright © 2022 Luo, Cruz-Ochoa, Seng, Egger, Lukacsovich, Lukacsovich and Földy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Neuritin Mediates Activity-Dependent Axonal Branch Formation in Part via FGF Signaling.J Neurosci. 2016 Apr 20;36(16):4534-48. doi: 10.1523/JNEUROSCI.1715-15.2016. J Neurosci. 2016. PMID: 27098696 Free PMC article.
-
[Correlation between hippocampal mossy fiber sprouting and synaptic reorganization and mechanisms of temporal lobe epilepsy].Zhonghua Yi Xue Za Zhi. 2007 Jan 30;87(5):341-4. Zhonghua Yi Xue Za Zhi. 2007. PMID: 17456365 Chinese.
-
Short-Term Depression of Sprouted Mossy Fiber Synapses from Adult-Born Granule Cells.J Neurosci. 2017 Jun 7;37(23):5722-5735. doi: 10.1523/JNEUROSCI.0761-17.2017. Epub 2017 May 11. J Neurosci. 2017. PMID: 28495975 Free PMC article.
-
The Molecular and Cellular Mechanisms of Axon Guidance in Mossy Fiber Sprouting.Front Neurol. 2018 May 29;9:382. doi: 10.3389/fneur.2018.00382. eCollection 2018. Front Neurol. 2018. PMID: 29896153 Free PMC article. Review.
-
Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: an emergent property of a complex system.Prog Brain Res. 2007;163:541-63. doi: 10.1016/S0079-6123(07)63029-5. Prog Brain Res. 2007. PMID: 17765737 Review.
Cited by
-
Similar Presynaptic Action Potential-Calcium Influx Coupling in Two Types of Large Mossy Fiber Terminals Innervating CA3 Pyramidal Cells and Hilar Mossy Cells.eNeuro. 2023 Feb 6;10(2):ENEURO.0017-23.2023. doi: 10.1523/ENEURO.0017-23.2023. Print 2023 Feb. eNeuro. 2023. PMID: 36697256 Free PMC article.
-
Commissural dentate granule cell projections and their rapid formation in the adult brain.PNAS Nexus. 2023 Mar 20;2(4):pgad088. doi: 10.1093/pnasnexus/pgad088. eCollection 2023 Apr. PNAS Nexus. 2023. PMID: 37077887 Free PMC article.
-
Activation of feedforward wiring in adult hippocampal neurons by the basic-helix-loop-helix transcription factor Ascl4.PNAS Nexus. 2024 May 3;3(5):pgae174. doi: 10.1093/pnasnexus/pgae174. eCollection 2024 May. PNAS Nexus. 2024. PMID: 38711810 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

