The Polycomb group gene rnf2 is essential for central and enteric neural system development in zebrafish
- PMID: 36117635
- PMCID: PMC9475114
- DOI: 10.3389/fnins.2022.960149
The Polycomb group gene rnf2 is essential for central and enteric neural system development in zebrafish
Abstract
The development of central nervous system (CNS) and enteric nervous system (ENS) is under precise and strict control in vertebrates. Whether and how the Polycomb repressive complex 1 (PRC1) is involved in it remain unclear. To investigate the role of PRC1 in the nervous system development, using CRISPR/Cas9 technology, we have generated mutant zebrafish lines for the rnf2 gene which encodes Ring1b, the enzymatic component of the PRC1 complex. We show that rnf2 loss of function leads to abnormal migration and differentiation of neural crest and neural precursor cells. rnf2 mutant embryos exhibit aganglionosis, in which the hindgut is devoid of neurons. In particular, the formation of 5-HT serotonin neurons and myelinating glial cells is defective. Furthermore, ectopic expression of ENS marker genes is observed in forebrain of rnf2 mutant embryos. These findings suggest that the rnf2 gene plays an important role in the migration and differentiation of neural precursor cells, and its absence leads to abnormal development of ENS and CNS in zebrafish.
Keywords: CNS; ENS; PRC1; neural crest; neural precursor cells; rnf2.
Copyright © 2022 Feng and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
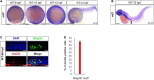
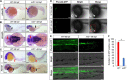
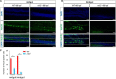





Similar articles
-
PRC1 Stabilizes Cardiac Contraction by Regulating Cardiac Sarcomere Assembly and Cardiac Conduction System Construction.Int J Mol Sci. 2021 Oct 21;22(21):11368. doi: 10.3390/ijms222111368. Int J Mol Sci. 2021. PMID: 34768802 Free PMC article.
-
Loss of the Polycomb group protein Rnf2 results in derepression of tbx-transcription factors and defects in embryonic and cardiac development.Sci Rep. 2019 Mar 13;9(1):4327. doi: 10.1038/s41598-019-40867-1. Sci Rep. 2019. PMID: 30867528 Free PMC article.
-
The polycomb group protein ring1b/rnf2 is specifically required for craniofacial development.PLoS One. 2013 Sep 11;8(9):e73997. doi: 10.1371/journal.pone.0073997. eCollection 2013. PLoS One. 2013. PMID: 24040141 Free PMC article.
-
Gut feelings: Studying enteric nervous system development, function, and disease in the zebrafish model system.Dev Dyn. 2018 Feb;247(2):268-278. doi: 10.1002/dvdy.24597. Epub 2017 Oct 23. Dev Dyn. 2018. PMID: 28975691 Review.
-
Emerging role of RNF2 in cancer: From bench to bedside.J Cell Physiol. 2021 Aug;236(8):5453-5465. doi: 10.1002/jcp.30260. Epub 2021 Jan 5. J Cell Physiol. 2021. PMID: 33400276 Review.
Cited by
-
Pcgf1 gene disruption reveals primary involvement of epigenetic mechanism in neuronal subtype specification in the enteric nervous system.Dev Growth Differ. 2023 Oct;65(8):461-469. doi: 10.1111/dgd.12880. Epub 2023 Aug 18. Dev Growth Differ. 2023. PMID: 37452641 Free PMC article.
-
Genetic regulation of enteric nervous system development in zebrafish.Biochem Soc Trans. 2024 Feb 28;52(1):177-190. doi: 10.1042/BST20230343. Biochem Soc Trans. 2024. PMID: 38174765 Free PMC article. Review.
-
The Contribution of the Zebrafish Model to the Understanding of Polycomb Repression in Vertebrates.Int J Mol Sci. 2023 Jan 24;24(3):2322. doi: 10.3390/ijms24032322. Int J Mol Sci. 2023. PMID: 36768643 Free PMC article. Review.
-
Haploinsufficiency of NFKBIA reshapes the epigenome antipodal to the IDH mutation and imparts disease fate in diffuse gliomas.Cell Rep Med. 2023 Jun 20;4(6):101082. doi: 10.1016/j.xcrm.2023.101082. Cell Rep Med. 2023. PMID: 37343523 Free PMC article.
-
Long-Term Implicit Epigenetic Stress Information in the Enteric Nervous System and its Contribution to Developing and Perpetuating IBS.Curr Neuropharmacol. 2024;22(13):2100-2112. doi: 10.2174/1570159X22666240507095700. Curr Neuropharmacol. 2024. PMID: 38726788 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

