Preparation and pharmacokinetics in vivo of linarin solid dispersion and liposome
- PMID: 36117666
- PMCID: PMC9476784
- DOI: 10.1016/j.chmed.2021.12.004
Preparation and pharmacokinetics in vivo of linarin solid dispersion and liposome
Abstract
Objective: The current investigation aimed to determine the appropriate dosage form by comparing solid dispersion and liposome to achieve the purpose of improving the solubility and bioavailability of linarin.
Methods: Linarin solid dispersion (LSD) and linarin liposome (LL) were developed via the solvent method and the thin film hydration method respectively. The Transwell chamber model of Caco-2 cells was established to evaluate the absorption of drug. The pharmacokinetics of linarin, LSD and LL in rats after ig administration were carried out by high performance liquid chromatography (HPLC) method.
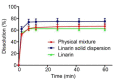
Results: The solubility of LSD and LL was severally 3.29 times and 3.09 times than that of linarin. The permeation coefficients of LSD and LL were greater than 10-6, indicating that the absorption of LSD and LL were both better than linarin. The bioavailability of the LSD was 3.363 times higher than that of linarin, and the bioavailability of LL was 0.9886 times higher than that of linarin.
Conclusion: The linarin was more suitable for making solid dispersion to enhance its solubility and bioavailability.
Keywords: bioavailabilit; intestinal absorption; linarin; liposome; pharmacokinetics; solid dispersion.
© 2022 Tianjin Press of Chinese Herbal Medicines. Published by ELSEVIER B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Effects of piperine on the intestinal permeability and pharmacokinetics of linarin in rats.Molecules. 2014 Apr 30;19(5):5624-33. doi: 10.3390/molecules19055624. Molecules. 2014. PMID: 24786847 Free PMC article.
-
Determination and pharmacokinetics of linarin in rat plasma after intramuscular administration of linarin solution and Yejuhua injection by HPLC.Biomed Chromatogr. 2015 Feb;29(2):164-6. doi: 10.1002/bmc.3269. Epub 2014 Jun 11. Biomed Chromatogr. 2015. PMID: 24917464
-
Amorphous solid dispersion of berberine with absorption enhancer demonstrates a remarkable hypoglycemic effect via improving its bioavailability.Int J Pharm. 2014 Jun 5;467(1-2):50-9. doi: 10.1016/j.ijpharm.2014.03.017. Epub 2014 Mar 5. Int J Pharm. 2014. PMID: 24607213
-
Improving Solubility and Bioavailability of Breviscapine with Mesoporous Silica Nanoparticles Prepared Using Ultrasound-Assisted Solution-Enhanced Dispersion by Supercritical Fluids Method.Int J Nanomedicine. 2020 Mar 10;15:1661-1675. doi: 10.2147/IJN.S238337. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32210559 Free PMC article.
-
Inventory of oral anticancer agents: Pharmaceutical formulation aspects with focus on the solid dispersion technique.Cancer Treat Rev. 2016 Nov;50:247-263. doi: 10.1016/j.ctrv.2016.09.012. Epub 2016 Sep 20. Cancer Treat Rev. 2016. PMID: 27776286 Review.
Cited by
-
Linarin and Hyperoside Inhibit lptD/msbA to Disrupt Membranes of Multidrug-Resistant Acinetobacter baumannii.Biology (Basel). 2025 Aug 20;14(8):1087. doi: 10.3390/biology14081087. Biology (Basel). 2025. PMID: 40906410 Free PMC article.
-
Tumor-Targeted cRGD-Coated Liposomes Encapsulating Optimized Synergistic Cepharanthine and IR783 for Chemotherapy and Photothermal Therapy.Int J Nanomedicine. 2024 Jun 19;19:6145-6160. doi: 10.2147/IJN.S457008. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38911506 Free PMC article.
-
Integrated metabolomic and transcriptomic analysis reveals biosynthesis mechanism of flavone and caffeoylquinic acid in chrysanthemum.BMC Genomics. 2024 Aug 3;25(1):759. doi: 10.1186/s12864-024-10676-6. BMC Genomics. 2024. PMID: 39097683 Free PMC article.
-
Simultaneous determination of multiple components in rat plasma by UHPLC-sMRM for pharmacokinetic studies after oral administration of Qingjin Yiqi Granules.Front Pharmacol. 2023 Apr 13;14:1155973. doi: 10.3389/fphar.2023.1155973. eCollection 2023. Front Pharmacol. 2023. PMID: 37124227 Free PMC article.
-
Recent nanoengineered therapeutic advancements in sepsis management.Front Bioeng Biotechnol. 2024 Dec 5;12:1495277. doi: 10.3389/fbioe.2024.1495277. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39703795 Free PMC article. Review.
References
-
- Chanput W., Krueyos N., Ritthiruangdej P. Anti-oxidative assays as markers for anti-inflammatory activity of flavonoids. International Immunopharmacology. 2016;40:170–175. - PubMed
-
- Choi J.S., Lee S.E., Jang W.S., Byeon J.C., Park J.S. Solid dispersion of dutasteride using the solvent evaporation method: Approaches to improve dissolution rate and oral bioavailability in rats. Materials Science & Engineering C, Materials for Biological Applications. 2018;90:387–396. - PubMed
-
- Das das Graças C de Souza, M., Cyrino, F. Z., de Carvalho, J. J., Blanc-Guillemaud, V., & Bouskela, E. (2018). Protective effects of micronized purified flavonoid fraction (MPFF) on a novel experimental model of chronic venous hypertension. European Journal of Vascular and Endovascular Surgery, 55(5), 694–702. - PubMed
-
- Feng X.C., Li Y., Guang C.X., Qiao M., Wang T., Chai L.W., Qiu F. Characterization of the in vivo and in vitro metabolites of linarin in rat biosamples and intestinal flora using ultra-high performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry. Molecules. 2018;23(9):2140. - PMC - PubMed
LinkOut - more resources
Full Text Sources

