Differential synaptic mechanism underlying the neuronal modulation of prefrontal cortex, amygdala, and hippocampus in response to chronic postsurgical pain with or without cognitive deficits in rats
- PMID: 36117908
- PMCID: PMC9478413
- DOI: 10.3389/fnmol.2022.961995
Differential synaptic mechanism underlying the neuronal modulation of prefrontal cortex, amygdala, and hippocampus in response to chronic postsurgical pain with or without cognitive deficits in rats
Abstract
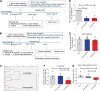
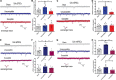
Chronic Postsurgical Pain (CPSP) is well recognized to impair cognition, particularly memory. Mounting evidence suggests anatomic and mechanistic overlap between pain and cognition on several levels. Interestingly, the drugs currently used for treating chronic pain, including opioids, gabapentin, and NMDAR (N-methyl-D-aspartate receptor) antagonists, are also known to impair cognition. So whether pain-related cognitive deficits have different synaptic mechanisms as those underlying pain remains to be elucidated. In this context, the synaptic transmission in the unsusceptible group (cognitively normal pain rats) was isolated from that in the susceptible group (cognitively compromised pain rats). It was revealed that nearly two-thirds of the CPSP rats suffered cognitive impairment. The whole-cell voltage-clamp recordings revealed that the neuronal excitability and synaptic transmission in the prefrontal cortex and amygdala neurons were enhanced in the unsusceptible group, while these parameters remained the same in the susceptible group. Moreover, the neuronal excitability and synaptic transmission in hippocampus neurons demonstrated the opposite trend. Correspondingly, the levels of synaptic transmission-related proteins demonstrated a tendency similar to that of the excitatory and inhibitory synaptic transmission. Furthermore, morphologically, the synapse ultrastructure varied in the postsynaptic density (PSD) between the CPSP rats with and without cognitive deficits. Together, these observations indicated that basal excitatory and inhibitory synaptic transmission changes were strikingly different between the CPSP rats with and without cognitive deficits.
Keywords: chronic postsurgical pain; cognitive function; excitatory postsynaptic currents (EPSCs); inhibitory postsynaptic synaptic currents (IPSCs); postsynaptic density.
Copyright © 2022 Li, He, Li, Sun, Zhang and Xiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
SCFAs Ameliorate Chronic Postsurgical Pain-Related Cognition Dysfunction via the ACSS2-HDAC2 Axis in Rats.Mol Neurobiol. 2022 Oct;59(10):6211-6227. doi: 10.1007/s12035-022-02971-8. Epub 2022 Jul 28. Mol Neurobiol. 2022. PMID: 35902549 Free PMC article.
-
The abused inhalant toluene differentially modulates excitatory and inhibitory synaptic transmission in deep-layer neurons of the medial prefrontal cortex.Neuropsychopharmacology. 2011 Jun;36(7):1531-42. doi: 10.1038/npp.2011.38. Epub 2011 Mar 23. Neuropsychopharmacology. 2011. PMID: 21430649 Free PMC article.
-
Group II mGluRs modulate baseline and arthritis pain-related synaptic transmission in the rat medial prefrontal cortex.Neuropharmacology. 2015 Aug;95:388-94. doi: 10.1016/j.neuropharm.2015.04.003. Epub 2015 Apr 22. Neuropharmacology. 2015. PMID: 25912637 Free PMC article.
-
Ageing, hippocampal synaptic activity and magnesium.Magnes Res. 2006 Sep;19(3):199-215. Magnes Res. 2006. PMID: 17172010 Review.
-
Making of a Synapse: Recurrent Roles of Drebrin A at Excitatory Synapses Throughout Life.Adv Exp Med Biol. 2017;1006:119-139. doi: 10.1007/978-4-431-56550-5_8. Adv Exp Med Biol. 2017. PMID: 28865018 Review.
Cited by
-
Hippocampal Extracellular Matrix Protein Laminin β1 Regulates Neuropathic Pain and Pain-Related Cognitive Impairment.Neurosci Bull. 2025 May 21. doi: 10.1007/s12264-025-01422-3. Online ahead of print. Neurosci Bull. 2025. PMID: 40397355
-
Exploring the influence of synaptic density and anxiety on pain perception: evidence from a [11C]UCB-J positron emission tomography imaging study.Int J Neuropsychopharmacol. 2025 Jul 23;28(7):pyaf040. doi: 10.1093/ijnp/pyaf040. Int J Neuropsychopharmacol. 2025. PMID: 40511849 Free PMC article.
References
LinkOut - more resources
Full Text Sources

