Altered levels of variant cholinesterase transcripts contribute to the imbalanced cholinergic signaling in Alzheimer's and Parkinson's disease
- PMID: 36117917
- PMCID: PMC9479005
- DOI: 10.3389/fnmol.2022.941467
Altered levels of variant cholinesterase transcripts contribute to the imbalanced cholinergic signaling in Alzheimer's and Parkinson's disease
Abstract
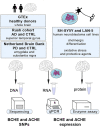
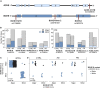
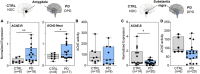
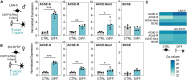
Acetylcholinesterase and butyrylcholinesterase (AChE and BChE) are involved in modulating cholinergic signaling, but their roles in Alzheimer's and Parkinson's diseases (AD and PD) remain unclear. We identified a higher frequency of the functionally impaired BCHE-K variant (rs1803274) in AD and PD compared to controls and lower than in the GTEx dataset of healthy individuals (n = 651); in comparison, the prevalence of the 5'-UTR (rs1126680) and intron 2 (rs55781031) single-nucleotide polymorphisms (SNPs) of BCHE and ACHE's 3'-UTR (rs17228616) which disrupt AChE mRNA targeting by miR-608 remained unchanged. qPCR validations confirmed lower levels of the dominant splice variant encoding the "synaptic" membrane-bound ACHE-S in human post-mortem superior temporal gyrus samples from AD and in substantia nigra (but not amygdala) samples from PD patients (n = 79, n = 67) compared to controls, potentially reflecting region-specific loss of cholinergic neurons. In contradistinction, the non-dominant "readthrough" AChE-R mRNA variant encoding for soluble AChE was elevated (p < 0.05) in the AD superior temporal gyrus and the PD amygdala, but not in the neuron-deprived substantia nigra. Elevated levels of BChE (p < 0.001) were seen in AD superior temporal gyrus. Finally, all three ACHE splice variants, AChE-S, AChE-R, and N-extended AChE, were elevated in cholinergic-differentiated human neuroblastoma cells, with exposure to the oxidative stress agent paraquat strongly downregulating AChE-S and BChE, inverse to their upregulation under exposure to the antioxidant simvastatin. The multi-leveled changes in cholinesterase balance highlight the role of post-transcriptional regulation in neurodegeneration. (235).
Keywords: Alzheimer’s disease; Parkinson’s disease; SNPs (single-nucleotide polymorphisms); acetylcholinesterase; butyrylcholinesterase; splice variants.
Copyright © 2022 Gok, Madrer, Zorbaz, Bennett, Greenberg, Bennett and Soreq.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Association A. S. (2019). 2019 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 15 321–387.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

