The association of antiviral drugs with COVID-19 morbidity: The retrospective analysis of a nationwide COVID-19 cohort
- PMID: 36117966
- PMCID: PMC9471091
- DOI: 10.3389/fmed.2022.894126
The association of antiviral drugs with COVID-19 morbidity: The retrospective analysis of a nationwide COVID-19 cohort
Abstract
Background and objectives: Although several repurposed antiviral drugs have been used for the treatment of COVID-19, only a few such as remdesivir and molnupiravir have shown promising effects. The objectives of our study were to investigate the association of repurposed antiviral drugs with COVID-19 morbidity.
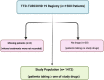
Methods: Patients admitted to 26 different hospitals located in 16 different provinces between March 11-July 18, 2020, were enrolled. Case definition was based on WHO criteria. Patients were managed according to the guidelines by Scientific Board of Ministry of Health of Turkey. Primary outcomes were length of hospitalization, intensive care unit (ICU) requirement, and intubation.
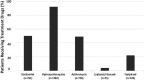
Results: We retrospectively evaluated 1,472 COVID-19 adult patients; 57.1% were men (mean age = 51.9 ± 17.7years). A total of 210 (14.3%) had severe pneumonia, 115 (7.8%) were admitted to ICUs, and 69 (4.7%) were intubated during hospitalization. The median (interquartile range) of duration of hospitalization, including ICU admission, was 7 (5-12) days. Favipiravir (n = 328), lopinavir/ritonavir (n = 55), and oseltamivir (n = 761) were administered as antiviral agents, and hydroxychloroquine (HCQ, n = 1,382) and azithromycin (n = 738) were used for their immunomodulatory activity. Lopinavir/ritonavir (β [95% CI]: 4.71 [2.31-7.11]; p = 0.001), favipiravir (β [95% CI]: 3.55 [2.56-4.55]; p = 0.001) and HCQ (β [95% CI]: 0.84 [0.02-1.67]; p = 0.046) were associated with increased risk of lengthy hospital stays. Furthermore, favipiravir was associated with increased risks of ICU admission (OR [95% CI]: 3.02 [1.70-5.35]; p = 0.001) and invasive mechanical ventilation requirement (OR [95% CI]: 2.94 [1.28-6.75]; p = 0.011).
Conclusion: Our findings demonstrated that antiviral drugs including lopinavir, ritonavir, and favipiravir were associated with negative clinical outcomes such as increased risks for lengthy hospital stay, ICU admission, and invasive mechanical ventilation requirement. Therefore, repurposing such agents without proven clinical evidence might not be the best approach for COVID-19 treatment.
Keywords: COVID-19 morbidity; ICU requirement; antiviral agents; invasive mechanical ventilation; length of hospitalization.
Copyright © 2022 Babayigit, Kokturk, Kul, Cetinkaya, Atis Nayci, Argun Baris, Karcioglu, Aysert, Irmak, Akbas Yuksel, Sekibag, Baydar Toprak, Azak, Mulamahmutoglu, Cuhadaroglu, Demirel, Kerget, Baran Ketencioglu, Ozger, Ozkan, Ture, Ergan, Avkan Oguz, Kilinc, Ercelik, Ulukavak Ciftci, Alici, Nurlu Temel, Ataoglu, Aydin, Cetiner Bahcetepe, Gullu, Fakili, Deveci, Kose, Tor, Gunluoglu, Altin, Turgut, Tuna, Ozturk, Dikensoy, Yildiz Gulhan, Basyigit, Boyaci, Oguzulgen, Borekci, Gemicioglu, Bayraktar, Elbek, Hanta, Kuzu Okur, Sagcan, Uzun, Akgun, Altinisik, Dursun, Cakir Edis, Gulhan, Oner Eyuboglu, Gultekin, Havlucu, Ozkan, Sakar Coskun, Sayiner, Kalyoncu, Itil and Bayram.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer BH declared a shared affiliation with the authors, II and AK to the handling editor at the time of review.
Figures
Similar articles
-
Safety and efficacy of antiviral combination therapy in symptomatic patients of Covid-19 infection - a randomised controlled trial (SEV-COVID Trial): A structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Oct 20;21(1):866. doi: 10.1186/s13063-020-04774-5. Trials. 2020. PMID: 33081849 Free PMC article. Clinical Trial.
-
Favipiravir Experience in COVID-19 Patients at a Tertiary Center Intensive Care Unit.Sisli Etfal Hastan Tip Bul. 2022 Jun 28;56(2):189-195. doi: 10.14744/SEMB.2021.35902. eCollection 2022. Sisli Etfal Hastan Tip Bul. 2022. PMID: 35990298 Free PMC article.
-
Comparing the impact of Hydroxychloroquine based regimens and standard treatment on COVID-19 patient outcomes: A retrospective cohort study.Saudi Pharm J. 2020 Dec;28(12):1877-1882. doi: 10.1016/j.jsps.2020.09.019. Epub 2020 Oct 1. Saudi Pharm J. 2020. PMID: 33020690 Free PMC article.
-
Impact of some antiviral drugs on health care utilization for patients with COVID-19: a systematic review and meta-analysis.Expert Rev Anti Infect Ther. 2023 Sep 5:1-17. doi: 10.1080/14787210.2023.2254491. Online ahead of print. Expert Rev Anti Infect Ther. 2023. PMID: 37667876 Review.
-
Common anti-COVID-19 drugs and their anticipated interaction with anesthetic agents.J Anaesthesiol Clin Pharmacol. 2021 Apr-Jun;37(2):160-170. doi: 10.4103/joacp.JOACP_461_20. Epub 2021 Jul 15. J Anaesthesiol Clin Pharmacol. 2021. PMID: 34349362 Free PMC article. Review.
Cited by
-
Cholesterol and COVID-19-therapeutic opportunities at the host/virus interface during cell entry.Life Sci Alliance. 2024 Feb 22;7(5):e202302453. doi: 10.26508/lsa.202302453. Print 2024 May. Life Sci Alliance. 2024. PMID: 38388172 Free PMC article. Review.
References
-
- World Health Organization. WHO coronavirus (covid-19) dashboard. Geneva: World Health Organization; (2021).
LinkOut - more resources
Full Text Sources

