Pre-existing anti-HCoV-OC43 immunity influences the durability and cross-reactivity of humoral response to SARS-CoV-2 vaccination
- PMID: 36118022
- PMCID: PMC9478943
- DOI: 10.3389/fcimb.2022.978440
Pre-existing anti-HCoV-OC43 immunity influences the durability and cross-reactivity of humoral response to SARS-CoV-2 vaccination
Abstract
Purpose: This study was conducted in order to properly understand whether prior seasonal human coronavirus (HCoV) immunity could impact the potential cross-reactivity of humoral responses induced by SARS-CoV-2 vaccine, thereby devising universal coronavirus vaccines for future outbreaks.
Methods: We performed enzyme-linked immunosorbent assay (ELISA) to quantify the immunoglobulin G (IgG) antibody levels to spike (S) protein and S1 subunit of HCoVs (HCoV-OC43, HCoV-HKU1, HCoV-NL63, and HCoV-229E), and ELISA [anti-RBD and anti-nucleoprotein (N)], chemiluminescence immunoassay assays (anti-RBD), pseudovirus neutralization test, and authentic viral neutralization test to detect the binding and neutralizing antibodies to SARS-CoV-2 in the vaccinees.
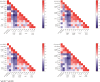
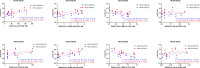
Results: We found that the antibody of seasonal HCoVs did exist before vaccination and could be boosted by SARS-CoV-2 vaccine. A further analysis demonstrated that the prior S and S1 IgG antibodies of HCoV-OC43 were positively correlated with anti-RBD and neutralization antibodies to SARS-CoV-2 at 12 and 24 weeks after the second vaccination, and the correlation is more statistically significant at 24 weeks. The persistent antibody levels of SARS-CoV-2 were observed in vaccinees with higher pre-existing HCoV-OC43 antibodies.
Conclusion: Our data indicate that inactivated SARS-CoV-2 vaccination may confer cross-protection against seasonal coronaviruses in most individuals, and more importantly, the pre-existing HCoV-OC43 antibody was associated with protective immunity to SARS-CoV-2, supporting the development of a pan-coronavirus vaccine.
Keywords: SARS-CoV-2; antibody; cross-reactivity; protective immunity; seasonal human coronavirus.
Copyright © 2022 Hu, Wang, Ren, Hao, Zhu, Jiang, Wang, Li and Shao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Are higher antibody levels against seasonal human coronaviruses associated with a more robust humoral immune response after SARS-CoV-2 vaccination?Front Immunol. 2022 Sep 8;13:954093. doi: 10.3389/fimmu.2022.954093. eCollection 2022. Front Immunol. 2022. PMID: 36159791 Free PMC article.
-
Cross-reactive antibody against human coronavirus OC43 spike protein correlates with disease severity in COVID-19 patients: a retrospective study.Emerg Microbes Infect. 2021 Dec;10(1):664-676. doi: 10.1080/22221751.2021.1905488. Emerg Microbes Infect. 2021. PMID: 33734013 Free PMC article.
-
Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination Boosts Neutralizing Activity Against Seasonal Human Coronaviruses.Clin Infect Dis. 2022 Aug 24;75(1):e653-e661. doi: 10.1093/cid/ciac057. Clin Infect Dis. 2022. PMID: 35079775 Free PMC article.
-
An overview on the seven pathogenic human coronaviruses.Rev Med Virol. 2022 Mar;32(2):e2282. doi: 10.1002/rmv.2282. Epub 2021 Aug 2. Rev Med Virol. 2022. PMID: 34339073 Review.
-
Evolutionary trajectory of SARS-CoV-2 and emerging variants.Virol J. 2021 Aug 13;18(1):166. doi: 10.1186/s12985-021-01633-w. Virol J. 2021. PMID: 34389034 Free PMC article. Review.
Cited by
-
SARS-CoV-2 mRNA Vaccines Induce Cross-Reactive Antibodies to NL63 Coronavirus but Do Not Boost Pre-Existing Immunity Anti-NL63 Antibody Responses.Vaccines (Basel). 2025 Mar 4;13(3):268. doi: 10.3390/vaccines13030268. Vaccines (Basel). 2025. PMID: 40266143 Free PMC article.
-
Enhanced Assessment of Cross-Reactive Antigenic Determinants within the Spike Protein.Int J Mol Sci. 2024 Jul 26;25(15):8180. doi: 10.3390/ijms25158180. Int J Mol Sci. 2024. PMID: 39125749 Free PMC article.
-
The role of cross-reactive immunity to emerging coronaviruses: Implications for novel universal mucosal vaccine design.Saudi Med J. 2023 Oct;44(10):965-972. doi: 10.15537/smj.2023.44.10.20230375. Saudi Med J. 2023. PMID: 37777266 Free PMC article. Review.
-
Early monitoring-to-warning Internet of Things system for emerging infectious diseases via networking of light-triggered point-of-care testing devices.Exploration (Beijing). 2023 Oct 5;3(6):20230028. doi: 10.1002/EXP.20230028. eCollection 2023 Dec. Exploration (Beijing). 2023. PMID: 38264687 Free PMC article.
-
Immunity Induced by Inactivated SARS-CoV-2 Vaccine: Breadth, Durability, Potency, and Specificity in a Healthcare Worker Cohort.Pathogens. 2023 Oct 18;12(10):1254. doi: 10.3390/pathogens12101254. Pathogens. 2023. PMID: 37887770 Free PMC article.
References
-
- Aguilar-Bretones M., Westerhuis B. M., Raadsen M. P., de Bruin E., Chandler F. D., Okba N. M., et al. . (2021). Seasonal coronavirus-specific b cells with limited SARS-CoV-2 cross-reactivity dominate the IgG response in severe COVID-19. J. Clin. Invest. 131 (21), e150613. doi: 10.1172/JCI150613 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

