Clinicopathologic Characteristics and Outcomes for Patients With KRAS G12D-Mutant NSCLC
- PMID: 36118132
- PMCID: PMC9471201
- DOI: 10.1016/j.jtocrr.2022.100390
Clinicopathologic Characteristics and Outcomes for Patients With KRAS G12D-Mutant NSCLC
Abstract
Introduction: Co-occurring mutations in KRAS-mutant NSCLC are associated with discrete biological properties and modulate therapeutic susceptibilities. As G12D-specific inhibitors are expected to enter the clinic, we sought to investigate the characteristics and outcomes of patients with KRAS G12D-mutant NSCLC.
Methods: This was a retrospective single-institution study. Patients with NSCLC and KRAS G12D mutations detected by the Massachusetts General Hospital SNaPshot next-generation sequencing assay were identified. Clinical and pathologic characteristics were collected by chart review.
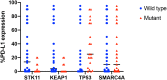
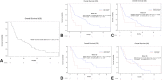
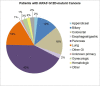
Results: A total of 107 patients with KRAS G12D-mutant NSCLC were identified. Most patients were former smokers (80, 74.8%) and had tumors with adenocarcinoma pathologic subtype (93, 86.9%). Among 56 patients evaluated for programmed death-ligand 1 expression, tumor proportion score was less than 50% in 43 (76.8%). Concomitant mutations were identified in STK11 (17 of 107, 15.9%), KEAP1 (10 of 58, 17.2%), TP53 (36 of 107, 33.6%), and SMARCA4 (11 of 107, 10.3%). Among 57 patients treated with first-line therapy, patients with STK11 co-mutations had shorter progression-free survival (1.2 mo, 95% confidence interval [CI]: 0.6-2.9 versus 4.1 mo, 95% CI: 2.5-6.0, p = 0.0235) and overall survival (4.3 mo, 95% CI: 1.2-10.6 versus 17.9 mo, 95% CI: 8.6-31.1, p = 0.0018) compared with wild type. Patients with KEAP1 co-mutations had shorter overall survival (4.6 mo, 95% CI: 1.2-10.6 versus 17.9 mo, 95% CI: 7.1-30.1, p = 0.0125) than those without. TP53 co-mutations exerted no influence on survival.
Conclusions: Co-occurring mutations were common in patients with KRAS G12D-mutant NSCLC. STK11 and KEAP1 co-mutations were associated with worse clinical outcomes, whereas co-occurring TP53 did not affect survival.
Keywords: Co-mutations; KRAS mutation; Non–small cell lung cancer; Targeted therapies.
© 2022 The Authors.
Figures


References
-
- Thai A.A., Solomon B.J., Sequist L.V., Gainor J.F., Heist R.S. Lung cancer. Lancet. 2021;398:535–554. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

