Tacrolimus Concentration Is Effectively Predicted Using Combined Clinical and Genetic Factors in the Perioperative Period of Kidney Transplantation and Associated with Acute Rejection
- PMID: 36118414
- PMCID: PMC9481373
- DOI: 10.1155/2022/3129389
Tacrolimus Concentration Is Effectively Predicted Using Combined Clinical and Genetic Factors in the Perioperative Period of Kidney Transplantation and Associated with Acute Rejection
Abstract
Background: Tacrolimus has unpredictable pharmacokinetic (PK) characteristics, which are partially attributed to CYP3A5 polymorphism. The potential effects of clinical factors in the postoperative period of transplantation on tacrolimus PK and those of early tacrolimus PK variability on clinical outcomes are yet to be clarified.
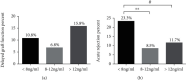
Methods: We examined the genetic and clinical factors affecting early tacrolimus PK variability in 256 kidney transplant recipients. The relationships among tacrolimus exposure, graft function delay (DGF), and acute rejection (AR) were further explored. Findings. The CYP3A5 genotype were strongly associated with tacrolimus concentration/dose ratio (C 0/D). Additionally, ABCB1 (rs1045642 and rs2032582) and ABCC2 (rs3740066) were found to have potential independent effects on early tacrolimus C 0/D in multivariate analysis. Red blood counts and albumin level were the most significant clinical factors associated with tacrolimus C 0/D. Wuzhi capsule also exerted an effect on tacrolimus PK. A model combined with pharmacogenetic and clinical factors explained 43.4% tacrolimus PK variability compared with 16.3% on the basis of CYP3A5 genotype only. Notably, increasing tacrolimus concentrations in the early postoperative stage were associated with AR, but not DGF.
Conclusions: Combined analysis of genotype and specific clinical factors is important for the formulation of precise tacrolimus dose regimens in the early stage after kidney transplantation.
Copyright © 2022 Fang Cheng et al.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


References
-
- First M. R. Tacrolimus based immunosuppression. Journal of Nephrology . 2004;17(Suppl 8):S25–S31. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

