Ion channel Piezo1 activation promotes aerobic glycolysis in macrophages
- PMID: 36119083
- PMCID: PMC9479104
- DOI: 10.3389/fimmu.2022.976482
Ion channel Piezo1 activation promotes aerobic glycolysis in macrophages
Abstract
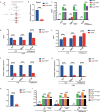
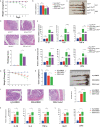
Altered microenvironmental stiffness is a hallmark of inflammation. It is sensed by the mechanically activated cation channel Piezo1 in macrophages to induce subsequent immune responses. However, the mechanism by which the mechanosensitive signals shape the metabolic status of macrophages and tune immune responses remains unclear. We revealed that Piezo1-deficient macrophages exhibit reduced aerobic glycolysis in resting or liposaccharide (LPS)-stimulated macrophages with impaired LPS-induced secretion of inflammatory cytokines in vitro. Additionally, pretreatment with the Piezo1 agonist, Yoda1, or cyclical hydrostatic pressure (CHP) upregulated glycolytic activity and enhanced LPS-induced secretion of inflammatory cytokines. Piezo1-deficient mice were less susceptible to dextran sulfate sodium (DSS)-induced colitis, whereas Yoda1 treatment aggravated colitis. Mechanistically, we found that Piezo1 activation promotes aerobic glycolysis through the Ca2+-induced CaMKII-HIF1α axis. Therefore, our study revealed that Piezo1-mediated mechanosensitive signals Piezo1 can enhance aerobic glycolysis and promote the LPS-induced immune response in macrophages.
Keywords: HIF1 alpha; Piezo1; colitis; glycolysis; macrophage.
Copyright © 2022 Leng, Zhang, Wang, Qin, Liu, Liu, Sheng, Feng, Hu and Peng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Owegi HO, Egot-Lemaire S, Waite LR, Waite GN. Macrophage activity in response to steady-state oxygen and hydrogen peroxide concentration - biomed 2010. BioMed Sci Instrum. (2010) 46:57–62. - PubMed
-
- Restrepo-Pineda S, Sánchez-Puig N, Pérez NO, García-Hernández E, Valdez-Cruz NA, Trujillo-Roldán MA. The pre-induction temperature affects recombinant HuGM-CSF aggregation in thermoinducible escherichia coli. Appl Microbiol Biotechnol (2022) 106(8):2883–902. doi: 10.1007/s00253-022-11908-z - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

