Dual Fc optimization to increase the cytotoxic activity of a CD19-targeting antibody
- PMID: 36119088
- PMCID: PMC9471254
- DOI: 10.3389/fimmu.2022.957874
Dual Fc optimization to increase the cytotoxic activity of a CD19-targeting antibody
Abstract
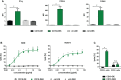
Targeting CD19 represents a promising strategy for the therapy of B-cell malignancies. Although non-engineered CD19 antibodies are poorly effective in mediating complement-dependent cytotoxicity (CDC), antibody-dependent cell-mediated cytotoxicity (ADCC) or antibody-dependent cellular phagocytosis (ADCP), these effector functions can be enhanced by Fc-engineering. Here, we engineered a CD19 antibody with the aim to improve effector cell-mediated killing and CDC activity by exchanging selected amino acid residues in the Fc domain. Based on the clinically approved Fc-optimized antibody tafasitamab, which triggers enhanced ADCC and ADCP due to two amino acid exchanges in the Fc domain (S239D/I332E), we additionally added the E345K amino acid exchange to favor antibody hexamerization on the target cell surface resulting in improved CDC. The dual engineered CD19-DEK antibody bound CD19 and Fcγ receptors with similar characteristics as the parental CD19-DE antibody. Both antibodies were similarly efficient in mediating ADCC and ADCP but only the dual optimized antibody was able to trigger complement deposition on target cells and effective CDC. Our data provide evidence that from a technical perspective selected Fc-enhancing mutations can be combined (S239D/I332E and E345K) allowing the enhancement of ADCC, ADCP and CDC with isolated effector populations. Interestingly, under more physiological conditions when the complement system and FcR-positive effector cells are available as effector source, strong complement deposition negatively impacts FcR engagement. Both effector functions were simultaneously active only at selected antibody concentrations. Dual Fc-optimized antibodies may represent a strategy to further improve CD19-directed cancer immunotherapy. In general, our results can help in guiding optimal antibody engineering strategies to optimize antibodies' effector functions.
Keywords: ADCC; ADCP; CD19; CDC; Fc engineering; antibody hexamerization; antibody therapy.
Copyright © 2022 Gehlert, Rahmati, Boje, Winterberg, Krohn, Theocharis, Cappuzzello, Lux, Nimmerjahn, Ludwig, Lustig, Rösner, Valerius, Schewe, Kellner, Klausz and Peipp.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

